Abstract
Echocardiography may miss prosthetic heart valve (PHV) endocarditis which advocates for novel imaging techniques to improve diagnostic accuracy and patient outcome. The purpose of this study was to determine the complementary diagnostic value of cardiac computed tomography angiography (CTA) to the clinical routine workup including transthoracic and transesophageal echocardiography (TTE/TEE) in patients with suspected PHV endocarditis and its impact on patient treatment. A diagnostic prospective cross-sectional study was chosen as design. Besides clinical routine workup (including TTE/TEE), CTA was performed to assess its diagnostic accuracy and complementary diagnostic/therapeutic value. For the diagnostic accuracy, the reference standard was surgical findings or clinical follow-up. To determine the complementary diagnostic/therapeutic value an expert-panel was used as reference standard. Twenty-eight patients were included. CTA resulted in a major diagnostic change in six patients (21 %) mainly driven by novel detection of mycotic aneurysms by CTA. Furthermore, treatment changes occurred in seven patients (25 %) compared to clinical routine workup. Diagnostic accuracy of routine clinical workup plus CTA was superior to clinical routine workup alone for the detection of PHV endocarditis in general, vegetations and peri-annular extension. This study demonstrates that CTA and clinical workup including TTE and TEE are complementary in patients with PHV endocarditis. Therefore, CTA imaging has to be considered after clinical routine workup in patients with a high suspicion on PHV endocarditis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prosthetic heart valve (PHV) endocarditis is a life-threatening disease with an incidence of 0.3–1.2 % per patient year [1]. Patients can present with a broad spectrum of symptoms such as fever, sepsis, heart failure symptoms, new or changing murmur or signs of systematic embolization but may be absent. Therefore, PHV endocarditis is a difficult diagnosis to establish based on these clinical symptoms alone. The modified Duke criteria [2], in which echocardiography plays a key role, are used to establish a definite or possible diagnosis of PHV endocarditis. The two major Duke criteria are positive blood cultures and a positive echocardiogram for signs of PHV endocarditis [2]. The first criterion is often negative (23–37 %) in patients with definite PHV endocarditis [3–5]. For that reason, reliable echocardiography is even more important to establish the definite diagnosis in patients with suspected PHV endocarditis [2].
Non-invasive transthoracic echocardiography (TTE) is the first line imaging modality to detect signs of PHV endocarditis. However, TTE often fails to detect vegetations, novel or increased paravalvular regurgitation and mycotic aneurysms/abscesses as signs of PHV endocarditis [3, 4, 6]. After TTE imaging, semi-invasive transesophageal echocardiography (TEE) is routinely performed. TEE has a good sensitivity and specificity but still misses life-threatening complications such as mycotic aneurysms and abscesses in up to 30 % of patients [3–5, 7–15]. This is mainly caused by acoustic shadowing of the PHV which obscures adjacent anatomical structures and hampers diagnostic assessment. These mycotic aneurysms and abscesses are not rare (53–55 %) in patients with PHV endocarditis and are associated with a high mortality (30–54 %) [4, 8]. The detection of these life-threatening mycotic aneurysms and abscesses with non or semi-invasive imaging is therefore crucial to initiate timely surgical intervention in order to improve clinical outcome [16].
Cardiac computed tomography angiography (CTA) is a promising novel non-invasive imaging technique to evaluate patients with (suspected) PHV endocarditis [7, 17–19]. At present, no clinical studies are available which investigate the complementary diagnostic value of CTA in patients with suspected PHV endocarditis and its implications for treatment strategy.
The aim of this study was to determine the complementary diagnostic value of cardiac CTA to the clinical routine workup (including TTE and TEE) in patients with suspected PHV endocarditis and its implications on treatment strategy.
Methods
Study design
A prospective diagnostic cross-sectional study was designed in the tertiary setting. This study has been approved by the institutional review board (IRB) [IRB number 10-008] and informed consent was obtained from all patients.
Study population
From May 2010 until July 2012, patients aged 18 years or older with suspected PHV endocarditis were enrolled. PHV endocarditis was suspected on the basis on clinical symptoms, physical examination, electrocardiogram and laboratory testing. These patients were referred for TTE and TEE to detect signs of PHV endocarditis. Patients who underwent both TTE and TEE as part of the routine clinical workup were eligible for inclusion. Six patients with renal impairment [glomerular filtration rate (GFR) <45] were excluded. After obtaining informed consent, additional CTA imaging was performed in 28 patients.
Study population characteristics
The following patient characteristics were prospectively collected: age, gender, the presence of fever, congestive heart failure, sepsis, systemic embolization to vital organs and initiated antibiotic treatment before clinical presentation. Further, PHV characteristics (PHV position, type of prosthesis and date of implantation) were collected. Physical examination was performed during clinical presentation and the following parameters were collected: blood pressure, heart rate, and the presence of endocarditis stigmata and new or changed murmur on auscultation. Laboratory testing included blood culture testing, C-reactive protein level, leukocyte count, creatinine and GFR. The electrocardiogram at clinical presentation was assessed for new AV-blocks.
Transthoracic and transoesophageal echocardiography
TTE and TEE was performed with state-of-the-art probes and image acquisition was performed according the clinical guidelines [1, 7, 20]. Echocardiographic evaluation focused on the detection of signs of PHV endocarditis: vegetations, new or increased paravalvular leakage, mycotic aneurysms or abscesses and PHV dehiscence. These signs were defined according to the ESC guidelines as follows: (1) vegetations, defined as irregularly shaped, oscillating or non-oscillating masses, adherent to and distinct from the myocardium; (2) abscesses, defined as irregularly shaped, inhomogeneous paravalvular enclosed masses within the peri-annular region, myocardium or pericardium; (3) mycotic aneurysms or pseudoaneurysms were defined as echo-free perivalvular cavities with flow communicating with the cardiovascular lumen; and (4) paravalvular leakage, defined as blood flow outside the PHV ring with or without rocking motion [1, 7, 20].
Cardiac computed tomography angiography
CT acquisition
Cardiac computed tomography angiography (CTA) was preferably performed within 3 days after TEE. Patients underwent CTA imaging preferably on a 256-slice CT system or alternatively on a 64-slice system (iCT and Brilliance 64, respectively, Philips Medical Systems, Cleveland, OH). After a scout view, a unenhanced prospectively ECG-triggered acquisition of the PHV region only was performed with the following acquisition parameters: 120 kV, 30 mAs, collimation 128 × 0.625, gantry rotation time 270 ms and pitch 0 for the 256-slice CT system. The acquisition parameters for the 64-slice CT system were: 120 kV, 55 mAs, collimation 64 × 0.625, gantry rotation 0.40 and pitch 0. Data were reconstructed (slice thickness 0.9 mm, increment 0.45) for the 75 % phase of the ECG-interval with filtered back projection. Subsequently, a contrast-enhanced retrospectively ECG-gated CT acquisition was performed with the following parameters: 120 kV, 600–700 mAs, collimation 64 or 128 × 0.625, gantry rotation time 420 or 270–330 ms and pitch 0.20 or 0.16–0.18. Gantry rotation time and pitch were dependent on heart rate.
A dual (400 mg jopromide/ml) or triphasic (300 ml jopromide/ml) contrast administration protocol was used. A locator was placed in the descending aorta. When the threshold of 100 HU was reached, data acquisition was initiated after a post-threshold delay of 8 s. The mean flow rate was set to 5.0–6.7 cc/s. Total contrast volume for the triphasic injection protocol was dependent on patient body weight (BW), scan duration and the added delay. Iodine flow varied between 1.6 (BW <70 kg), 1.8 (BW 70–85 kg) and 2.0 gram (BW >85 kg) iodine/s. In the first phase, only contrast medium was injected. Secondly, a mixture of 30 % contrast medium and 70 % saline is administered followed by a saline flush. For the dual phase injection protocol, 100 cc contrast was followed by a saline flush. Added delay varied between 6 and 8 s. The effective radiation dose was estimated from the product of the total dose-length product indicated by the scanner (including all parts of image acquisition) and a conversion coefficient (k = 0.0145 mSv/[mGyx cm]) [21].
Image reconstruction
Data were reconstructed (slice thickness 0.9 mm, increment 0.45) equally spaced for each 10 % interval of the ECG-interval resulting in 11 datasets (including an additional 75 % ECG-phase). Images were transferred to a clinical workstation and analyzed using dedicated software (Extended Brilliance Workstation, Philips Medical Systems, Philips, Best, the Netherlands). Diastolic and systolic imaging data sets were reconstructed in orthogonal imaging planes (in plane, parallel and perpendicular to the prosthetic valve) and used for image evaluation. Additional reconstructions similar to the standard echocardiographic views were reconstructed if needed as well from the same CT datasets. During analysis, it is important to differentiate between vegetations and beam-hardening artefacts because both are hypodense. However, artefacts and vegetations can be differentiated based on the fact that vegetations are often oval and irregular circumscribed hypodense abnormalities and beam-hardening artifacts are linear within the direction of the beam.
Reference standard and outcome measures
Complementary value of cardiac CTA to clinical routine workup
An expert-panel was used to determine the additional diagnostic value of cardiac CTA and its impact on treatment strategy (Fig. 1). The expert-panel consisted of two cardiac surgeons, two cardiologists and two radiologists with an interest in PHV imaging.
In the expert-panel consensus meeting, each case was presented in the following sequence: (1) clinical routine workup (clinical history, physical examination, laboratory testing, TTE and TEE) and followed by (2) the cardiac CTA examination. After each of the two assessment moments, the expert-panel determined a consensus on the diagnosis and treatment strategy (Fig. 1). A standardized scoring form was used. The primary outcome measures were (1) the complementary diagnostic value of CTA to clinical workup in patients with suspected PHV endocarditis and (2) its impact on treatment strategy. Major and minor diagnostic changes caused by CTA were distinguished. Major diagnostic change was defined as the novel detection of a vegetation or abscess/mycotic aneurysm by CTA. In case of abscesses and/or mycotic aneurysms absent on echocardiography, a major diagnostic change was scored if CTA detected an abscess or mycotic aneurysm. Minor diagnostic change was defined as detection of an increased number and/or size of peri-annular extensions (mycotic aneurysms/abscesses) or better depiction of the relationship of the peri-annular extension with relevant cardiac structures such as coronary arteries compared to clinical routine workup. A major treatment strategy change based on CTA was defined as conversion from conservative to surgical treatment or visa versa. Minor treatment strategy change was defined as change of surgical strategy (i.e. aortic valve replacement vs. aortic allograft implantation).
Diagnostic accuracy
The diagnostic accuracy was determined for PHV endocarditis in general, vegetations and peri-annular complications (mycotic aneurysms/abscesses). PHV endocarditis in general was defined as any positive imaging sign of PHV endocarditis (vegetations, new or increased paravalvular leakage and peri-annular complications). Vegetations and peri-annular complications were defined in the echocardiography section. The reference standard used to determine diagnostic accuracy were surgical, microbiological and/or pathological findings or in patients treated conservatively clinical follow-up (at least 1 month). Successful conservative treatment was defined as uncomplicated clinical follow-up with unchanged TTE examination. Diagnostic accuracy was determined for the clinical routine workup (including both TTE and TEE) and clinical routine workup plus CTA.
Data-analysis
Data-analysis was performed in SPSS software (version 15). Continuous variables are presented as mean ± standard deviation (SD) or medians and interquartile range (IQR) dependent on the data distribution. Parametric data distribution was assessed with QQ-plots and Kolmogorov–Smirnov test. Categorical variables are presented in numbers (percentages). Diagnostic and therapeutic changes are expressed in numbers and percentages. Diagnostic accuracy measures (sensitivity, specificity, positive and negative predictive values) including 95 % confidence intervals (CI) were calculated.
Results
Patient population
Twenty-eight patients with a high suspicion of PHV endocarditis were included in this prospective diagnostic cross-sectional study. Relevant study population characteristics are given in Table 1. In this study population with a high suspicion of PHV endocarditis, blood cultures were positive in 16 (57 %) patients and the modified Duke criteria were met in 17/28 (61 %) patients. Cardiac CTA examinations were performed on 256-slice (n = 26) and 64—slice CT systems (n = 2). Median radiation exposure was 11.8 mSv (IQR 11.2–12.8). The diagnosis of the clinical routine workup (including both TTE and TEE) and clinical routine workup plus CTA is presented per patient in Table 2. Median interval between TEE and CTA was 0 days (IQR 0–1 day, range 0–17 days). In this population, 16 of the 28 patients (57 %) underwent reoperation and these patients were positive according to the modified Duke criteria in 11 (69 %) of the cases. Median interval between cardiac CTA and reoperation was 14 days (IQR 6-70). The other 11 (43 %) patients, who were Duke positive in 50 % of the cases, were treated successfully with antibiotics. In the study population, the median clinical follow-up was 5.5 (IQR 3–9) months with 89 % (n = 25) endocarditis free survival. One patient (number 24, Table 2) (4 %) died in hospital after surgical treatment. Two patients (number 22/28, Table 2) had recurrence of PHV endocarditis in the re-operated group: one patient was re-operated twice and one patient was treated successfully with antibiotics.
Complementary value of cardiac CTA to clinical routine workup
Diagnostic change
In six out of the 28 (21 %) patients, CTA resulted in a major diagnostic change which was confirmed by surgical exploration in five (83 %) patients. The other patient (number 20, Table 2) was treated successfully with antibiotics. These major diagnostic changes included detection of four additional mycotic aneurysms (RCC n = 2; LCC n = 2) and two vegetations. MDCT imaging also provided information that resulted in minor additional diagnostic changes in 13/28 (46 %) patients (Table 2).
Treatment strategy change
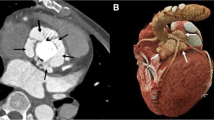
The treatment strategy changed after CTA compared to clinical routine workup in 7 out of 28 (25 %) patients. In one patient (number 17, Table 2), CTA detected a large mycotic aneurysm (former LCC, Fig. 2) which was missed by clinical routine workup. This changed the treatment strategy from intravenous antibiotic treatment to urgent surgical exploration with implantation of an allograft and therefore was classified as major treatment change. In the other six patients, only the surgical strategy changed (minor treatment change): additional aortic root surgery instead of only PHV replacement (allograft in 3, Bentall procedure in 2 and addition of a pericardial patch in 1).
Mycotic aneurysm mainly located near the former left cusp. Patient 17 with a biological Carpentier Edwards Perimount PHV in the aortic position. Assessment of the former left coronary cusp region is hampered by acoustic shadowing (arrows) in the short axis TEE view (a) and 0 degree TEE view (c). Short axis (b) and 0 degree MDCT (D) images are not hampered by valve-related artifacts and visualized a large (3.4 × 2.1 cm) mycotic aneurysm (arrows). PHV prosthetic heart valve, TEE transesophageal echocardiography
Diagnostic accuracy
In Supplement 1 and 2, diagnostic accuracy data for PHV endocarditis in general, vegetations and peri-annular complications (mycotic aneurysm/abscesses) are presented.
PHV endocarditis in general
Sensitivity and specificity for PHV endocarditis in general were 95 and 83 % for the routine clinical workup (including TTE/TEE). For clinical routine workup plus CTA, sensitivity and specificity for PHV endocarditis in general increased to 100 and 83 %, respectively. In the false positive patient (number 5, Table 2), routine workup and the routine workup plus CTA detected one mycotic aneurysm located at the former NCC that was not confirmed during surgical exploration. In the other 15 re-operated patients (94 %), imaging findings were confirmed by surgical exploration. In one patient (number 18, Table 2), the routine workup missed a vegetation which was detected by MDCT and confirmed at surgery (Table 2). However, time interval between TEE and MDCT was 17 days in this patient.
Vegetations
Sensitivity and specificity for the detection of vegetations were 63/100 % for clinical routine workup and 100/100 % for clinical routine workup plus CTA, respectively. Echocardiography missed three vegetations in patients with biological (n = 2, Fig. 3) and mechanical PHVs (n = 1). In one patient (number 20, Fig. 4) surgical exploration was not performed (Table 2).
Detection of a vegetation by MDCT. Patient with an allograft in the aortic position. The short axis TEE view demonstrated a tricuspid aortic valve with central mal-coaptation and no evidence of a vegetation (a). Color doppler imaging (b) revealed a moderate central aortic regurgitation. The short axis MDCT image demonstrated a vegetation on left coronary cusp (c). MDCT multidetector-row computed tomography, TEE transesophageal echocardiography
Detection of a vegetation by MDCT. Patient with a Mitroflow bioprosthesis in the aortic position. The short axis TEE did not show a vegetation (a). The MDCT short axis view demonstrated a vegetation on the former left coronary cusp (arrow) (b). MDCT multidetector-row computed tomography, TEE transesophageal echocardiography
Peri-annular complications
Eighteen patients had 26 peri-annular complications (mycotic aneurysms n = 23; abscesses n = 3) (Table 2). Sensitivity and specificity were 68/91 % for the clinical workup and 100/91 % for the clinical workup plus CTA, respectively. Routine clinical workup missed eight mycotic aneurysms mainly located around former RCC (n = 5) which were correctly detected by CTA (Fig. 5). However, CTA also missed three abscesses which were detected by TEE and confirmed by surgery located around the left coronary artery (Fig. 6) and non-coronary cusp in two patients (number 24/27, Table 2). The clinical workup (including TTE and TEE) detected all abscesses and CTA detected all mycotic aneurysms resulting in a total sensitivity of 100 % combining all imaging modalities.
Mycotic aneurysm located near the former right coronary cusp. Patient 8 with a mechanical bileaflet St Jude PHV in the aortic position. Assessment of the former right coronary cusp is hampered by acoustic shadowing (arrows) in the short axis TEE view (a) and 0 degree TEE view (c). Short axis (b) and 0 degree MDCT (d) images are not hampered by valve-related artifacts and visualized a mycotic aneurysm (arrows). MDCT multidetector-row computed tomography, PHV prosthetic heart valve, TEE transesophageal echocardiography
Abscess formation in PHV endocarditis. Patient 24 with a bileaflet mechanical St Jude PHV in the aortic position. A short axis TEE supravalvular view demonstrated two echolucent cavities (asterisk) around the left coronary artery without color flow (b) suggestive for abscess formation. The color flow is present in the left coronary artery. Short axis supravalvular MDCT image demonstrates aortic wall thickening as an aspecific sign of aortitis but did not visualize the abscesses. The two abscesses were confirmed during surgical exploration. MDCT multidetector-row computed tomography, PHV prosthetic heart valve, TEE transesophageal echocardiography
Discussion
Our study demonstrates the additional diagnostic value of cardiac CTA to clinical routine workup (including TTE and TEE) and its impact on treatment strategy. Furthermore CTA detected all mycotic aneurysms (n = 8) that were missed by echocardiography, whereas echocardiography detected all abscesses (n = 3), which were missed by CTA. CTA also provided additional diagnostic information on the extensiveness of the mycotic aneurysms that was valuable for the surgical planning.
Complementary value of cardiac CTA to clinical routine workup
Cardiac CTA imaging resulted in a major diagnostic change compared to the routine clinical workup in 21 % of patients and resulted in a change of treatment strategy in 25 % of patients: in one patient it converted medical treatment to urgent surgery and in six others it changed the surgical strategy. Besides treatment strategy change, cardiac CTA can provide in patients considered for reoperation additional information on the presence of coronary artery disease, the presence and location of coronary bypassgrafts, calcifications in the ascending aorta (cross-clamping) and the relationship between coronary arteries and peri-annular extension. Most PHV types do not hamper coronary assessment [22]. In patients with sufficient image quality of coronary arteries invasive conventional coronary angiography may be omitted.
Diagnostic accuracy
In this study, this clinical workup had a good sensitivity/specificity (95/83 %) to establish the diagnosis of PHV endocarditis mainly in patients with aortic PHVs (n = 25). This is in line with previous publications [4–6, 23, 24]. Sensitivity raised to 100 % after addition of a complementary CTA examination. In three patients, clinical routine workup missed the vegetation which was detected by CTA. However, in two of these three patients the predefined time interval (≤3 days) from clinical workup to CTA was exceeded. In the subgroup analyses without these two patients, the sensitivity of the clinical routine workup increased substantially from 63 to 83 % for the detection of vegetations. However, the sensitivity of the clinical routine workup plus CTA remained 100 % in this group.
In PHV endocarditis is the detection of peri-annular complications of paramount importance as it is with a high mortality compared to uncomplicated PHV endocarditis and requires surgical treatment. Our study shows that for the detection of peri-annular complications clinical workup and cardiac CTA are complementary. Echocardiography detected all abscesses (n = 3) which were missed by CTA. The reason for the false negative CTA findings is probably the absence of contrast in enclosed masses (e.g. abscesses). In retrospect, aspecific aortic wall thickening was present on the abscess locations detected with echocardiography. In contrary, CTA detected all mycotic aneurysms (n = 8) that were missed by echocardiography. Importantly this was the first and only sign of peri-annular extension in four patients resulting in a major diagnostic change (Table 2). The mycotic aneurysms missed by echocardiography in patients with aortic PHVs were mainly located in the former RCC region (anterior side of aortic root). Diagnostic assessment of this region is often hampered by acoustic shadowing during TEE examination. The complementary approach (combining echocardiography and CTA) did not fail to identify peri-annular complications and had therefore a sensitivity of 100 % in the detection of complicated PHV endocarditis.
Previous studies on the value of cardiac CTA in the evaluation of PHV endocarditis are scarce [7, 18]. Fagman et al. [18] compared CTA to TEE in twenty-seven patients with aortic PHV endocarditis. Sixteen patients underwent surgical exploration. In contrast to our study, the conservatively treated group (n = 11) was not included in the analysis resulting in a selection bias. This study also found that cardiac CTA and TEE are complementary in the detection in peri-annular extension. However, Fagman et al. [18] did not examine the complementary value of cardiac CTA to the normal clinical routine workup but compared CTA to TEE (replacement design). Furthermore, this study provided no insights on treatment strategy change in patients with PHV endocarditis. Feuchtner et al. [7] evaluated the value of CTA in a small patient population (n = 6) with suspected PHV endocarditis. This study also compared CTA to TEE instead of using an add-on design. Furthermore, in this study also only re-operated patients were included resulting in a selection bias. This study found that CTA better depicted the extensiveness of mycotic aneurysms which is in line with our results.
Limitations
First, 12 patients did not undergo surgical exploration. However, in the non-operated patients clinical follow-up data was collected. This is the methodology to analyze the clinically relevant suspected population. Second, median interval (14 days) between imaging and surgical reoperation was relatively long. However, no novel pathological findings were found during surgical exploration Third, CTA evaluation has some disadvantages namely radiation exposure and administration of iodinated contrast agents. In this patient population with concomitant high mortality and morbidity, these risks are defendable. Fourth, five potential study participants were not enrolled because of renal impairment (GFR <45) and is important for the clinical implementation of CTA in patients with suspected PHV endocarditis. At last, a relatively small number of patients (n = 28) were included resulting in large confidence intervals for diagnostic accuracy measures.
In conclusion, this study demonstrates that cardiac CTA and clinical workup including TTE and TEE are complementary to establish the diagnosis of PHV endocarditis and to detect peri-annular complications. Cardiac CTA imaging resulted in a major diagnostic change compared to the routine clinical workup in 21 % of patients and results in a change of treatment strategy in 25 % of patients, Therefore, we advise to include cardiac CTA imaging in the diagnostic workup of every patient with suspected PHV endocarditis.
References
Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I et al (2009) Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 30:2369–2413
Durack DT, Lukes AS, Bright DK (1994) New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 96:200–209
Choussat R, Thomas D, Isnard R, Michel PL, Iung B, Hanania G et al (1999) Perivalvular abscesses associated with endocarditis; clinical features and prognostic factors of overall survival in a series of 233 cases. Perivalvular Abscesses French Multicentre Study. Eur Heart J 20:232–241
Daniel WG, Mugge A, Martin RP, Lindert O, Hausmann D, Nonnast-Daniel B et al (1991) Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N Engl J Med 324:795–800
San Roman JA, Vilacosta I, Sarria C, de la Fuente L, Sanz O, Vega JL et al (1999) Clinical course, microbiologic profile, and diagnosis of periannular complications in prosthetic valve endocarditis. Am J Cardiol 83:1075–1079
Mugge A, Daniel WG, Frank G, Lichtlen PR (1989) Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol 14:631–638
Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H et al (2009) Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 53:436–444
Hill EE, Herijgers P, Claus P, Vanderschueren S, Peetermans WE, Herregods MC (2007) Abscess in infective endocarditis: the value of transesophageal echocardiography and outcome: a 5-year study. Am Heart J 154:923–928
Karalis DG, Bansal RC, Hauck AJ, Ross JJ Jr, Applegate PM, Jutzy KR et al (1992) Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis. Clinical and surgical implications. Circulation 86:353–362
Khandheria BK, Seward JB, Oh JK, Freeman WK, Nichols BA, Sinak LJ et al (1991) Value and limitations of transesophageal echocardiography in assessment of mitral valve prostheses. Circulation 83:1956–1968
Leung DY, Cranney GB, Hopkins AP, Walsh WF (1994) Role of transoesophageal echocardiography in the diagnosis and management of aortic root abscess. Br Heart J 72:175–181
Mohr-Kahaly S, Kupferwasser I, Erbel R, Wittlich N, Iversen S, Oelert H et al (1993) Value and limitations of transesophageal echocardiography in the evaluation of aortic prostheses. J Am Soc Echocardiogr 6:12–20
Pedersen WR, Walker M, Olson JD, Gobel F, Lange HW, Daniel JA et al (1991) Value of transesophageal echocardiography as an adjunct to transthoracic echocardiography in evaluation of native and prosthetic valve endocarditis. Chest 100:351–356
Taams MA, Gussenhoven EJ, Bos E, de Jaegere P, Roelandt JR, Sutherland GR et al (1990) Enhanced morphological diagnosis in infective endocarditis by transoesophageal echocardiography. Br Heart J 63:109–113
Gussenhoven EJ, Taams MA, Roelandt JR, Ligtvoet KM, McGhie J, van Herwerden LA et al (1986) Transesophageal two-dimensional echocardiography: its role in solving clinical problems. J Am Coll Cardiol 8:975–979
Habib G, Tribouilloy C, Thuny F, Giorgi R, Brahim A, Amazouz M et al (2005) Prosthetic valve endocarditis: who needs surgery? A multicentre study of 104 cases. Heart 91:954–959
Habets J, Budde RP, Symersky P, van den Brink RB, de Mol BA, Mali WP et al (2011) Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol 8:466–478
Fagman E, Perrotta S, Bech-Hanssen O, Flinck A, Lamm C, Olaison L et al (2012) ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 22:2407–2414
Habets J, Tanis W, Mali WP, Chamuleau SA, Budde RP (2012) Imaging of prosthetic heart valve dysfunction: complementary diagnostic value of TEE and MDCT? JACC Cardiovasc Imaging 5:956–961
Sachdev M, Peterson GE, Jollis JG (2003) Imaging techniques for diagnosis of infective endocarditis. Cardiol Clin 21:185–195
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
Habets J, van den Brink RB, Uijlings R, Spijkerboer AM, Mali WP, Chamuleau SA et al (2012) Coronary artery assessment by multidetector computed tomography in patients with prosthetic heart valves. Eur Radiol 22:1278–1286
Daniel WG, Mugge A, Grote J, Hausmann D, Nikutta P, Laas J et al (1993) Comparison of transthoracic and transesophageal echocardiography for detection of abnormalities of prosthetic and bioprosthetic valves in the mitral and aortic positions. Am J Cardiol 71:210–215
Lowry RW, Zoghbi WA, Baker WB, Wray RA, Quinones MA (1994) Clinical impact of transesophageal echocardiography in the diagnosis and management of infective endocarditis. Am J Cardiol 73:1089–1091
Acknowledgments
We thank K.A. van Rijnbach for her help with the final edition of the tables, figures and flowcharts. This study was supported by a grant of the Netherlands Heart Foundation [Grant number 2009B014].
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jesse Habets and Wilco Tanis have contributed equally to this work and shared first authorship.
Steven A. J. Chamuleau and Ricardo P. J. Budde have contributed equally to this work and shared last authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Habets, J., Tanis, W., van Herwerden, L.A. et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 30, 377–387 (2014). https://doi.org/10.1007/s10554-013-0335-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-013-0335-2