Abstract
Inflammatory-proteolytic processes in the vessel wall are essential in the pathophysiology of abdominal aortic aneurysm (AAA). It has been demonstrated that, 18F-FDG-PET/CT may be useful for detection of pathological wall metabolism and therefore risk stratification. Quantification of the FDG-uptake in AAA wall is hampered by partial-volume (PV)-effects. For correction and accurate quantitative 18F-FDG-uptake analysis we designed and validated a novel IDL-based software in correlation to phantom studies, histopathology and clinical presentation of AAA patients. For in vivo studies 23 patients with symptomatic and asymptomatic AAA underwent 18F-FDG-PET/CT before surgery. In areas with 18F-FDG-uptake the maximum and mean standardized uptake values in the vessel wall with (PVC-SUVmax, PVC-SUVmean) and without (SUVmax, SUVmean) PV-correction were determined. Results were correlated with clinical presentation, corresponding macrophage-infiltration and MMP-2- and -9-expression in surgical specimens. In patients, SUVmax, SUVmean as well as PVC-SUVmax or PVC-SUVmean enabled a highly significant (p < 0.005) discrimination of symptomatic and asymptomatic AAA. Uncorrected and corrected SUVs showed comparable correlations with macrophage-infiltration and MMP-9 expression. No correlation of 18F-FDG-uptake and MMP-2 was found. In vivo correlations of detected FDG-uptake with clinical and histological results showed comparable results for corrected and uncorrected SUVs. PV-correction is not mandatory for qualitative clinical assessment of glucose metabolism in the vessel wall of AAA-patients but may be necessary to establish quantitative cut off values to stratify patients for aneurysm repair.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abdominal aortic aneurysm (AAA) is a pathological dilatation of the infra-renal aorta with an overall incidence of 5–8 % in man over 65 years. Prevalence of therapy needing AAA with a maximum diameter of more than 5.5 cm and indication for surgery is 1–2 % in the male population over 60 years [1]. Left untreated AAA > 5.5 cm will rupture in 40–60 % of cases within 5 years [2] leading to a total mortality of more than 90 %. However, prophylactic surgical AAA repair has also high mortality rates [1–4]. Therefore, surgeons have to weight out the risk of rupture against the risks of surgical intervention. In clinical practice, the surgical decision making is commonly based on aneurysm size. A maximum diameter > 5.5 cm is a well accepted criterion for operative repair. However, using this criterion, some unexpected ruptures of smaller aneurysms may occur while many larger AAA may be stable for a long time period and the patient will be exposed unnecessarily to the potential harms of prophylactic surgery [5–7].
This insufficient prognostic reliability is caused by the fact that predominant destabilizing pathophysiological processes in the AAA wall as chronic inflammation and hyper-proteolysis leading finally to AAA rupture are not reflected by geometrical parameters and are not detected by conventional CT-imaging.
Interestingly, it has been reported that these destabilizing inflammatory-proteolytic processes in the aortic wall may be visualized by 2-deoxy-2-[18F]fluoro-d-glucose (FDG-PET/CT) [8]. Thereby, increased FDG-uptake is correlated to higher local MMP tissue activities, extracellular matrix degradation as well as with clinical signs of wall instability as AAA progression and acute symptoms [8–10]. Thus, [18F]-FDG-metabolic imaging seems to be a potential new approach for risk stratification of AAA. However, accurate quantitative assessment of FDG-uptake in AAA wall so far, is hampered by several technical problems. The thickness of the aortic wall is 1–2 mm and is therefore smaller than the spatial resolution of routine PET-scanners leading to partial volume (PV) effects in quantitative analyses. Thereby, by luminal spill over effects from circulating unspecific [18F]-FDG-activity in the vessel lumen may result in falsely increased [18F]-FDG-uptake in AAA wall, while spill out effects of vascular lesions with intensive [18F]-FDG-uptake may reduce the measured signal intensity. For correction of these PV-effects we developed therefore a new software tool for accurate quantitative FDG-uptake analysis in AAA wall. Further, SUV measurements were validated in patients by correlation to clinical presentation and underlying histopathology in corresponding operatively retrieved tissue specimen out of AAA wall.
Materials and methods
Ethics
The study was approved by the Ethics Committee of the “Technische Universitaet” Muenchen. Written consent was available from all patients.
Phantom studies
For validation of our software we used a newly developed radioactive wax [11] model of the aorta. The vessel wall was made of radioactive wax containing 18F. The blood pool and background activity were simulated with 13N and 18F in aqueous solution. The two different isotopes were used to simulate various blood/wall (BW) ratios, due to the different half-life periods. 6 aortic phantoms with different wall thickness ranging from 5 to 20 mm were built and measured in the PET-CT scanner with the same imaging acquisition parameters as described below. Using the software described below, we determined recovery factors with and without PV-correction for the different wall thickness as well as for different BW-ratios. Recovery factors were determined based on the segmented tissue classes in CT (lumen, wall, and thrombus). A geometrical model of the vessel was calculated for each individual case. To remove the spill in from the blood pool, the data of this model was blurred using the spatial resolution of the PET scanner. Meaning we simulated how the blood pool would influence the uptake in the wall, this amount was then subtracted from the wall activity in the corresponding area. PVC was performed in the same way: the geometrical model of the wall was used to estimate the partial volume effect based on the spatial resolution of the scanner by blurring the CT model with this spatial resolution. In phantom studies it was found that without PV-correction SUVs measured were in average 65 % of the real activity present in the phantom. In contrast, SUVs corrected for PV-effects were in average 92 % of the activity present in the phantom.
Patients
Twenty-three patients scheduled for open AAA-repair were included in the study. Sixteen patients were male, seven female. Eighteen patients were asymptomatic and five were symptomatic. Fifteen of these patients were analyzed by us recently (8). Symptomatic aneurysms are defined by sudden onset of severe, steady, and worsening middle abdominal and back pain due to growth of the aneurysm. The average age of the patients was 72 years (range 59–87). More details about the study population are shown in Table 1.
PET/CT imaging
Patients were examined with a Siemens Biograph TruePoint 64 PET/CT (Siemens Medical Solutions, Erlangen, Germany) after injection of [18F]-FDG (5 MBq/kg body weight). Imaging was performed 90 min after injection of the tracer as described (8). Patients fasted at least 6 h before the PET examination and the blood glucose level was <150 mg/dl in all patients. PET data was reconstructed using an attenuation-weighted ordered subsets expectation maximization algorithm (AW-2D-OSEM). Attenuation- and scatter correction was performed based on low-dose-CT data acquired before the PET acquisition. After the PET/CT standard protocol CT angiographies (120 kV, 250 mAs; Imeron 400 MCT, Bracco Imaging, Germany) were performed. The arrival of the contrast dye in the aorta was individually achieved by bolus tracking. 0.6 mm axial slices were reconstructed in 3 mm slices.
Data analysis
For comparative analyses acquired PET/CT data were transferred to the new in-house progammed software system and routinely to the commercial Syngo workstation with TrueD software (Siemens Medical Systems, Malvern, PA, USA). Thereby images were evaluated together by a nuclear medicine physician and a vascular surgeon. In each AAA the region with maximum focal 18F-FDG-uptake in the vessel wall was localized and for later comparative studies defined by PET/CT coordinates. For precise intra-operative tissue sampling these areas were mapped also in relation to the renal arteries and the aortic bifurcation while orientation in the cross sectional plane was defined clockwise. In TrueD analyses of a 1 × 0.5 cm2 elliptic ROI was placed over these areas with accentuated 18F-FDG and the dosage and body weight adjusted maximum and average uncorrected standard uptake values (SUVmax/SUVmean) were measured. For PV corrected analysis the fused PET/CT data slices an in-house programmed software using IDL (Interactive Data Language, ITT Visual Information Solution, Boulder, CO, USA) was used. In this software tool, the lumen of the aorta as well as the aortic wall and if present a thrombus is segmented semi-automatically in the CT data. Based on these morphological information corrections for partial volume effects are performed: a geometrical model was constructed from the vessel contours using the measured maximal PET signal in the lumen. For blood pool removal, this model was blurred with the PET tomograph's spatial resolution and subtracted from the measured PET data. Activity from outside the vessel was ignored. In the next step the values in these new PET images were corrected for partial volume effects based on the blurred CT wall data. Quantitative PET and CT data were generated circumferentially in 100 chords determining mean, standard deviation and maximal signals. To simplify further statistical analysis, the chords were averaged into ten segments. Figure 1 shows a typical segmentation of the aorta by our software. For the analyses the PV-corrected maximum SUV (PVC-SUVmax) as well as the PV-corrected mean SUV (PVC-SUVmean) in the segment analog to the segment used for analyses in the TrueD software was estimated.
Tissue collection and Immunohistochemistry (IHC)
For assessment of MMP-2 and -9 tissue expression and quantification of macrophages in AAA vessel wall specimens were operatively retrieved from all patients during conventional AAA repair. Resection specimens were taken from the localization estimated before in the image analysis with the highest 18F-FDG uptake and used for quantification. Subsequent to resection specimens were dissected of luminal thrombus and peripheral tissue, fixed in 4 % paraformaldehyde and embedded in paraffin. For immunohistochemistry, paraffin sections were dewaxed in xylene and rehydrated through graded ethanol (100–70 %) to water. Primary antibody solution was added for 1 h at room temperature. MMP epitopes were detected using specific monoclonal antibody for the individual MMPs, followed by addition of second enzyme-linked antibody specific for the same MMP. Following the primary antibody incubation, visualisation was performed using ChemMate Detection Kit (LSAB, DakoCytomation) according the manufacturer’s instructions. All sections were evaluated by 3 experienced observers and semi-quantification was performed using a score of 0–6+, with 1+ indicating weakly positive, 2+ indicating more positive, 3+ indicating intermediate positive, 4+ indicating markedly positive, 5+ indicating strongly positive and 6+ indicating massive positive staining.
Results
Quantitative 18F-FDG-PET/CT in patients with AAA
We analyzed the 18F-FDG uptake in the vessel wall of 23 AAA patients and determined SUVmax, SUVmean, partial volume corrected (PVC)-SUVmax as well as PVC-SUVmean. PVC-SUVmax was significantly higher than the uncorrected SUVmax (6.6 ± 4.0 vs. 3.2 ± 1.7; p < 0.0001) in the total group of AAA patients as well as the PVC-SUVmean to the uncorrected SUVmean (4.6 ± 3.7 vs. 1.6 ± 1.2). Symptomatic AAAs are defined by sudden onset of severe, steady, and worsening middle abdominal and back pain due to expansion of the aneurysm. Comparing patients with symptomatic AAA (n = 6) and asymptomatic AAA (n = 18) we found significantly higher values in symptomatic patients compared to the asymptomatic patients for SUVmax, SUVmean, PVC-SUVmax, and PVC-SUVmean (Table 2). These data are consistent with the hypothesis that in patients with symptomatic AAA and high risk of rupture a significantly higher glucose metabolism is present in the vessel wall of the aneurysm.
Correlation of tracer uptake in the vessel wall with histological parameters
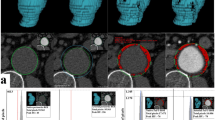
For correlation of SUV and tissue expression of MMP-2, -9 and macrophage infiltration with 18F-FDG-uptake in the vessel wall of AAAs paired data sets (histology and PET/CT) were available from the 23 patients who received conventional open AAA repair. Macrophage infiltration as well as tissue expressions of MMP-2 and -9 in operatively retrieved AAA wall samples were analysed semi-quantitatively as described above. We determined the correlation of histological parameters in the vessel wall with SUVmax, SUVmean, PVC-SUVmax, as well as PVC-SUVmean. Performing a regression analysis we found a statistically significant linear correlation of SUV and macrophage infiltration. R2 was 0.81, 0.78, 0.77 and 0.79 for SUVmax, SUVmean, PVC-SUVmax or PVC-SUVmean, respectively. We also found a linear correlation of these SUVs with MMP-9-expression. R2 was 0.63, 0.58, 0.59 and 0.58, respectively for SUVmax, SUVmean, PVC-SUVmax or PVC-SUVmean (Fig. 3). In contrast we did not find a linear correlation of SUV and MMP-2-expression. The correlation coefficients are shown in Table 3. Representative PET/CT-images and histology sections from a symptomatic and an asymptomatic AAA-patient are shown in Fig. 2. Regression curves for the correlation of the SUVs with macrophage infiltration as well as with the MMP-9 expression can be found in Fig. 3.
Discussion
It has been proposed that vascular 18F-FDG uptake in the vessel wall reflects pathological inflammatory changes [8–10]. Although previous publications report about a correlation of vascular FDG uptake with the risk of AAA progression and rupture so far definitive quantitative assessment of glucose metabolism in the vessel wall is still hampered by methodical problems. For this purpose we created a new software tool specially designed for quantification of 18F-FDG-uptake in aortic vessel wall. After segmentation and definition of AAA wall, thrombus and lumen the software allows partial volume correction as well as subtraction of spill over of blood pool activity. The new software was validated experimentally in a wax phantom of the aorta with variable vessel wall-, lumen- and background-activities. Furthermore, we studied the value of the software tool in vivo and compared PV corrected measurements in comparison to commercial software (Siemens TrueD) and underlying histology. In particular, we investigated the correlation of MMP-expression and SUV in AAA wall. Using commercial software we demonstrated recently that increased 18F-FDG uptake in the vessel wall of AAA patients correlates with macrophage-infiltration and increased MMP-9 expression [8, 9]. It is widely accepted that hyperproteolysis and macrophage infiltration correlates with growth and rupture risk of AAA [12]. Therefore, 18F-FDG-PET/CT seems to be a promising new approach for risk stratification in AAA by imaging of destructive inflammatory and proteolytic degradation, independent from morphological parameters as maximum diameter. A prerequisite for assessment of risk would be the definition of quantitative cut-off values. In our in vivo studies we analysed the 18F-FDG uptake in 23 AAA patients and found that FDG uptake helped to discriminate asymptomatic from potentially rupture prone symptomatic AAA, reliably. In addition, a strong statistical correlation of SUV with macrophage-infiltration (R2 = 0.8) and MMP-9-expression (R2 = 0.6) in the vessel wall of AAA patients for all examined SUV parameters was recognized as previously described in animal models for macrophage infiltration [13]. Surprisingly, experimentally validated quantitative SUV measurements did not improve discrimination of symptomatic and asymptomatic AAA and the correlation of FDG uptake with macrophage-infiltration and MMP-9, respectively. The finding that the partial-volume correction does not improve the correlation coefficients may be partly explained by the fact, that the aortic wall thickness had to be assumed as constant with 2 mm for all patients, because AAA wall cannot be quantified in CT- slices. Hence the predominant correction factor of our software tool the partial volume correction may be only of linear effect. Therefore, correlation coefficients as well as clinical discrimination seem to be not affected relevantly by PVC. Nevertheless, PVC is essential if absolute SUV values are of interest in particular if absolute cut-off values for therapy decisions should be established in the future for e.g. in multi-centre studies when multiple PET/CT systems with different spatial resolutions may be utilized. Therefore, in this context and for scientific issues PV corrected quantitative SUV analyses are of high clinical importance while for daily routine diagnostics PVC of AAA may be of minor interest. However, the presented data should interpreted with respect to some limitations. First, we study a rather small collective of AAA patients including 30 % female patients. This high percentage of females may not entirely be representative of the global population of AAA. Second, the imaging protocol is critical. We examined our patients 90 min after injection of FDG using the same protocol applied for oncologic patients. It has been reported that for assessment of vascular FDG-uptake a circulation time of 180 min is optimal [14]. This longer circulation time is critical to achieve in patients with acute AAA symptoms who need care on an intensive care unit. Third, as mentioned above estimation of aortic wall thickness and partial volume in conventional CT-imaging is hardly possible, our results may be hampered by use of isoforme AAA wall thickness. In the future more individual assumptions for AAA wall may be facilitated by MRI/PET machines with higher contrast and spatial resolution. Furthermore, FDG-uptake signal intensity was correlated with the underlying histopathology. Such quantitative correlation of FDG-uptake with the degree of tissue alterations has already been described for several inflammatory diseases, but not so far in AAA. Most of the aortic wall specimen showed inhomogeneous distribution of the main pathological characteristics so we used semi-quantitative visual evaluation as most integrative analysis. However, these correlations may be improved by full quantitative methods such as ELISA studies. Another technical limitation of our study is that sequential image acquisition of PET- and CT-data does not warrant perfect co-registration as movement of the aorta e.g. due to pulse waves or bowel motion is not taken into account, potentially leading to segmentation of structures surrounding the aorta and consequently to SUVs not representing aortic glucose metabolism. Though, in AAA-patients, uncorrected as well as corrected FDG-uptake analyses facilitated discrimination of symptomatic from asymptomatic AAA. Furthermore, we found significant correlation of FDG-signal intensity with tissue inflammation and MMP-proteolysis. Correlations to histology were not improved by PV-corrections. Nevertheless, PVC seems essential if absolute SUV values are of interest for comparative clinical studies in the future. After definition of such cut-of values FDG-PET/CT might be a new diagnostic tool for risk stratification of AAA.
References
Bengtson H, Sonesson B, Bergquist D (1996) Incidence and prevalence of abdominal aortic aneurysms estimated by necropsy studies and population screening by ultrasound. Ann N Y Acad Sci 800:1–24
Patel MI, Hardmann DT, Fisher CM et al (1995) Current views on the pathogenesis of abdominal aortic aneurysms. J Am Coll Surg 181:371–382
Vorp DA, Vande Geest JP (2005) Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol 2:1558–1566
Sakalihasan N, Limet R, Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365:1577–1589
Chaikof EL, Brewster DC, Dalman RL et al (2009) The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 50(4 Suppl):S2–S49
Brewster DC (2001) Presidential address: what would you do if it were your father? Reflections on endovascular abdominal aortic aneurysm repair. J Vasc Surg 33:1139–1147
Conrad MF, Crawford RS, Pedraza JD et al (2007) Long-term durability of open abdominal aortic aneurysm repair. J Vasc Surg 46:669–675
Reeps C, Essler M, Pelisek J et al (2008) Increased [18F]fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg 48:417–423
Reeps C, Gee MW, Maier A et al (2009) Glucose metabolism in the vessel wall correlates with mechanical instability and inflammatory changes in a patient with a growing aneurysm of the abdominal aorta. Circ Cardiovasc Imaging 2:507–509
Defawe OD, Hustinx R, Defraigne JO et al (2005) Distribution of F-18 fluorodeoxyglucose (F-18 FDG) in abdominal aortic aneurysm: high accumulation in macrophages seen on PET imaging and immunohistology. Clin Nucl Med 30:340–341
Bazañez-Borgert M, Bundschuh RA, Herz M et al (2008) Radioactive spheres without inactive wall for lesion simulation in PET. Z Med Phys 18:37–42
Choke E, Cockerill G, Wilson WR et al (2005) A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 30:227–244
Turner GH, Olzinski AR, Bernard RE et al. (2009) Assessment of macrophage infiltration in a murine model of abdominal aortic aneurysm. J Magn Reson Imaging 30:455–460
Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711
Acknowledgments
The study was funded in line with the budget of the institutional departments.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christian Reeps and Ralph A. Bundschuh contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Reeps, C., Bundschuh, R.A., Pellisek, J. et al. Quantitative assessment of glucose metabolism in the vessel wall of abdominal aortic aneurysms: correlation with histology and role of partial volume correction. Int J Cardiovasc Imaging 29, 505–512 (2013). https://doi.org/10.1007/s10554-012-0090-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0090-9