Abstract
Although many echocardiographic studies are available about the adaptation of left ventricle to intensive training, right heart function has been poorly investigated and no data are available about the right atrial (RA) function in top-level athletes. The aim of the study was to investigate RA function and dimension by standard echocardiography and 2D speckle tracking echocardiography (STE). One hundred top-levels athletes were recruited from professional sports team and were compared with 78 normal subjects. Athletes during an off-training period or during prolonged forced rest resulting from injuries were excluded. Top-level athletes had higher BSA as compared with controls and, as expected, a lower resting heart rate (p ≤ 0.001). RA area, volume, and volume index were significantly greater in athletes than in controls (p ≤ 0.001). This increase was associated with greater right ventricular and inferior vena cava diameters (p ≤ 0.001). Peak atrial longitudinal strain and peak atrial contraction strain values were significantly lower in athletes in comparison with controls (40.92 ± 9.86 % vs. 48.00 ± 12.68 %, p ≤ 0.001; 13.05 ± 4.84 % vs. 15.99 ± 5.74 %, p ≤ 0.001, respectively). Interestingly, while athletes presented a higher E/A ratio (p ≤ 0.001) and a lower peak A velocity (p ≤ 0.001), the E/e′ ratio did not differ between the two groups. In top-level athletes the RA presents a physiological adaptation to intensive exercise conditioning which determines not only a morphological but also a functional remodeling. We reported for the first time reference values of RA strain in elite athletes, demonstrating that 2D STE is a useful tool to investigate RA longitudinal myocardial deformation dynamics in athlete’s heart.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A high-intensity training program is associated with hemodynamic and morphological changes including increases in left ventricular (LV) chamber size, wall thickness, and mass, globally described as “athlete’s heart” [1–3]. Although there are many echocardiographic studies concerning LV structure and function, right heart function has been poorly investigated in the context of athlete’s heart. Because of the complex anatomy and the nonconcentric contraction of the right heart, the echocardiographic quantitative assessment of right function and geometry has been neglected for many years [4]. However, even if novel echocardiographic techniques have demonstrated promising results in the assessment of right ventricular (RV) function [4], to date, only a few studies have focused on the assessment of right heart in athletes, reporting increased dimension of RV and right atrium (RA) in athletes as compared with control subjects [5–7]. We previously reported that cardiac remodeling associated with long-term training determines a significant changing in the LV diastolic function and in LV filling period with a resulting typical pattern of left atrial longitudinal myocardial deformation dynamics in top-level athletes, as assessed by two-dimensional speckle tracking echocardiography (2D STE) [8]. Similarly, considering the relevant contribution of the RA in filling the RV and the peculiar remodeling demonstrated in the right heart of top-level athletes, we hypothesized that highly trained athletes could present a peculiar pattern of RA strain. Two-dimensional STE has been recently demonstrated as a feasible technique for the assessment of myocardial RA deformation dynamics with a good reproducibility [9]. However, to date, no echocardiographic research has focused on mechanical assessment of RA function per se in athletes. In this study we sought to determine for the first time RA function and dimension in top-level athletes engaged in different sports by standard echocardiography and by 2D STE. Moreover, we assessed RV diastolic function by pulsed-wave Doppler and by Doppler tissue imaging (TDI).
Methods
Study population
From August 2009 to September 2011, 110 top-level athletes were referred to our Echo laboratory of the University of Siena for a diagnostic examination. All athletes participating in the study were members of professional sports team (male soccer players of Siena Football Club, Italian Premier League; male basketball players of Mens Sana Basket Siena, Italian League professional basketball club; female volleyball players of Santa Croce Volleyball Club, Italian volleyball League of A2 series), all high ranking national or international competitors. All the athletes were engaged in an intensive training program, for at least 15 h/week (distributed in 6–8 sessions) for at least 5 years. They were submitted to training sessions at workloads ranging from 70 to 95 % of maximal HR as indicated by individual heart rate monitoring applied during the sessions. Training sessions mainly consisted of technical–tactical drills, running and sprinting conditioning (for football and basketball players) or jumping and sprinting conditioning for volleyball players. Athletes also performed 2 resistance training sessions per week at moderate workload under the supervision of a dedicated coach. From this group, all athletes meeting the inclusion and exclusion criteria were enrolled. We also studied a control group of 78 sedentary age- and sex-matched subjects without detectable cardiovascular disease and/or risk factors. None of the subjects in the control group had cardiovascular structural of functional abnormalities or received any medication. Subjects in the control group did not participate in competition sports and were engaged only in recreational activities, without following any kind of routine training program. All participants were asymptomatic and did not present family history of cardiac disease or sudden cardiac death. The athletes during an off-training period or during prolonged forced rest (>10 days) resulting from injuries were excluded from the study.
All participants underwent complete physical examination, electrocardiogram (ECG), standard echocardiography, and treadmill ECG test with no evidence of pathological findings. Body surface area (BSA) was calculated using the Dubois and Dubois formula [10].
After the rationale and the study protocol were explained, the participants gave written informed consent prior to their inclusion in the study. The investigational protocol was conformed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the institutional committee.
Echocardiographic measurements
Echocardiographic examination was performed by one cardiologist using a high-quality echocardiograph (Vivid 7, GE, USA), equipped with a 2.5 MHz probe. Subjects were studied in the steep left-lateral decubitus position. For all measurements, three beats were stored and analyzed off-line (EchoPac, GE, USA). Off-line data analysis was performed by one experienced reader, blinded to the clinical characteristics of the study population. All echocardiographic data were analyzed at the end of the data collection. Heart rate (HR) was measured from the electrocardiographic tracing taken during the echocardiographic examination.
RA dimension was measured from the apical 4-chamber view, at the end of LV systole (largest volume), excluding the area between the leaflets and annulus, following the RA endocardium, excluding the inferior vena cava (IVC) and superior vena cava and RA appendage, as recommended [11]. RA size was assessed by area and volume determinations. Although limited data are available for RA volumes, RA volume was determined for estimation of RA size, using the biplane method of discs formula, obtaining RA area and volume, then indexed to BSA [12]. RV end-diastolic chamber size was assessed using the measurement of the basal and mid cavity diameters from the apical 4-chamber view at end-diastole. In order to avoid an underestimation of RV width, an adjusted 4-chamber view was obtained to acquire the right ventricle-focused view, as recommended [11]. The IVC was examined from the subcostal view. The diameter of IVC was measured at 1.0–2.0 cm from the junction with the right atrium [12]. An M-mode cursor was placed through the tricuspid annulus, measuring the amount of longitudinal motion of the annulus at peak systole, to obtain tricuspid annular plane systolic excursion (TAPSE) value [11].
Pulsed-wave Doppler and tissue Doppler imaging
Pulsed-wave Doppler and TDI analyses were performed to determine RV diastolic function. The Doppler beam was aligned parallel to the RV inflow. The sample volume was placed at the tips of the tricuspid leaflets [11]. Care was taken to measure at held end-expiration taking an average of 3 consecutive beats. Doppler velocities of the transtricuspid flow (E, A, and E/A ratio) were obtained.
To perform TDI analysis, an apical 4-chamber was used, placing the sample volume at the tricuspid annulus. As recommended [11], care was taken to ensure optimal image orientation to avoid the underestimation of velocities. Tissue Doppler velocities of the tricuspid annulus (s′, e′, a′) and the derived parameters (e′/a′ ratio) were obtained. The tricuspid E/e′ ratio was calculated and was considered as a reliable parameter of RA pressure [11, 13–15].
Two-dimensional speckle tracking echocardiography
Two-dimensional STE analysis was obtained from an apical 4-chamber view, using conventional 2D gray-scale echocardiography, during breath hold for 3 s during normal breathing, avoiding Valsalva maneuver, as previously described [9]. Three consecutive heart cycles were recorded and averaged. The frame rate was set between 60 and 80 frames per second. The off-line analysis was performed by a single experienced and independent reader who was not directly involved in the image acquisition and had no knowledge of other echocardiographic parameters representing LV and left atrial structure and function, using a commercially available semi-automated 2D strain software (EchoPac, GE, USA). RA endocardial border was manually traced in 4-chamber view, thus delineating a region of interest (ROI), composed by 6 segments. Then, after the segmental tracking quality analysis and the eventual manual adjustment of the ROI, the longitudinal strain curves were generated by the software for each atrial segment. All measurements were taken from the onset of QRS complex, as previously described for left and right atria [9, 16, 17]. RA peak atrial longitudinal strain (PALS) and RA peak atrial contraction strain (PACS) were calculated by averaging values observed in all RA segments. RA PALS was used to estimate RA reservoir function, while RA PACS was used to measure the phase of RA active conduit in late diastole during atrial contraction. The RA time to peak longitudinal strain (TPLS) and the RA time to peak contraction strain (TPCS) were also obtained. The E/e’ ratio was used in conjunction with PALS to derive a non-invasive dimensionless parameter of RA stiffness, as reported for the left atrium [18, 19].
Interobserver and intraobserver variability was assessed in a previous study from our research group [9].
Statistical analysis
Data were expressed as median values, 5th and 95th percentile. Categorical variables were expressed as percentages. Considering that echocardiographic variables did not show a normal distribution, as assessed by the Kolmogorov–Smirnov test, the non-parametric test of Mann–Whitney for two groups was used to compare median values. Differences in proportions were established by the Chi-squared test. Post hoc ANOVA analysis with Student–Neuman–Keuls was performed among the three groups of athletes. Correlation analysis was performed using the Spearman and Pearson methods as appropriate for data distribution. In addition, a multivariate stepwise linear regression analysis was performed to identify independent determinants of RA volume index, RA PALS and PACS values, and RA TPLS and TPCS values. The following variables were tested: HR, RA volume index, RA volume, RA area, TAPSE, E/A ratio, e′/a′ ratio, E/e′ ratio, RV basal diameter, and RV mid cavity diameter.
A p value <0.05 was considered statistically significant. All the analyses were performed by SPSS 14.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
Results
Comparison between athletes and controls
Ten athletes were excluded from the study because echocardiographic evaluation was performed during off-training period or during prolonged forced rest. A final population of 100 top-level athletes (76 males, 24 females) was analyzed and compared with 78 age- and sex-matched sedentary subjects (50 males, 28 females). Baseline characteristics of the study participants were listed in Table 1. BSA significantly differed between the two groups, being greater in the athletes’ group (p ≤ 0.001). As expected, resting HR was significantly lower in the athletes as compared with sedentary subjects (p ≤ 0.001).
Echocardiographic and Doppler parameters are reported in Table 2. Athletes showed a greater RA area and a greater RA volume as compared with controls (p ≤ 0.001). The statistical significance between two groups was demonstrated even when RA volume was indexed to BSA, with athletes presenting a greater RA volume index in comparison with controls (26.96 ± 7.28 2 vs. 19.89 ± 4.99 ml/m2, respectively, p ≤ 0.001).
Top-level athletes showed also an increase in RV dimensions, with RV basal and middle diameters being significantly higher as compared with normal subjects (p ≤ 0.001). Moreover, in top-level athletes TAPSE and IVC diameter values were significantly higher than those of control group (p ≤ 0.001).
Right heart Doppler analysis demonstrated that athletes had a higher E/A ratio (2.0 ± 0.5 vs. 1.70 ± 0.38, respectively, p < 0.005) with a lower peak A velocity in comparison with controls (0.32 ± 0.08 vs. 0.39 ± 0.09 m/s, respectively, p ≤ 0.001), while TDI indexes did not substantially differ between the groups. Interestingly, tricuspid E/e′ ratio did not significantly differ between athletes and controls.
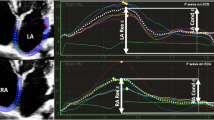
Two-dimensional STE parameters are reported in Table 3. Top-level athletes showed a peculiar pattern of RA myocardial deformation dynamics, with a significantly lower PALS value (40.92 ± 9.86 % vs. 48.00 ± 12.68 %, p ≤ 0.001) and a significantly lower PACS value (13.05 ± 4.84 % vs. 15.99 ± 5.74 %, p ≤ 0.001) as compared with normal subjects (Fig. 1). However, PALS/PACS ratio did not significantly differ between the two groups. Athletes showed also a peculiar time-to-peak pattern, being greater TPLS and TPCS values in the comparison between the two groups. Interestingly, RA stiffness, evaluated through the (E/e′)/PALS ratio, did not show significant differences between the two groups.
Right atrial longitudinal myocardial deformation dynamics by 2D speckle tracking echocardiography. A typical pattern of a top-level athlete compared with a typical pattern of a control subject. Dashed curve represents the average strain value. PALS peak atrial longitudinal strain, PACS peak atrial contraction strain, TPLS time to peak longitudinal strain, TPCS time to peak contraction strain
On multivariate analysis, in the population of athletes, RV E/A ratio was the only independent predictors of RA PALS (r = 0.45, β = 0.35, p < 0.01). RA volume index and RA area were the only independent predictors of TPLS (r = 0.36, p < 0.05, β = 0.55 and β = −0.67 respectively). HR, E/A ratio, E/e′, and RV basal diameter were the only independent predictors of TPCS (r = 0.84, p < 0.001, β = −0.77, 0.21, −0.32, and 0.24 respectively).
Comparison among different sporting disciplines
The whole athletic population was divided in three groups, according to the different sporting disciplines the athletes were involved: football, basketball, volleyball. All volleyball players were female, while all athletes practicing football and basketball were male. Demographic and echocardiographic characteristics of the athletes are reported in Table 4. Basketball players were older than football and volleyball players and exhibited a higher BSA, a greater IVC diameter, and greater right heart dimensional parameters in comparison with football and volleyball players. Football players showed greater RA and RV dimensions as compared with volleyball players. Interestingly, while E/e′ ratio, (E/e′)/PALS ratio, and PALS/PACS ratio did not substantially differ among the three groups, basketball players showed significantly lower values of RA PALS and PACS as compared with the other two groups, while RA strain parameters of football players were significantly lower in comparison with volleyball players.
Discussion
Many echocardiographic studies have investigated left heart size and function in athletes [1–3]; however few data concerning the right heart in athletes are available [5–7]. If the RV is the forgotten ventricle, RA has been ignored in the evaluation of cardiac function and adaptation with its assessment dwelling in true obscurity [17]. To date, no researches are available about the functional role of RA in the context of athlete’s heart. D’Andrea et al. [7] have recently documented that right heart measurements were significantly greater in elite endurance athletes than in age- and sex-matched strength athletes and controls. According to these previous published results, we demonstrated that top-level athletes show a greater RA size in the comparison with age- and sex-matched controls. Moreover, we reported for the first time RA volume measurements in top-level athletes demonstrating that RA size is significantly greater in athletes even when it was assessed by RA volume and the statistical significance remains also when RA volume is indexed to BSA.
Right heart, due to its thin wall, is known to be very sensitive to changes in preload and, while it is susceptible to elevated afterload, it better tolerates volume overload which is able to alter the geometry of right heart but not to influence the pattern of ejection [4, 11]. However, if sustained, volume overload will also ultimately lead to RV functional decline [4]. Athletes, particularly those engaged in endurance activity, exhibit greater cardiac dimensions in comparison with normal subjects, with a symmetry of training-induced dimensional changes affecting both the right and left heart and both the ventricles and atria [5–7]. In this study, we confirmed that the changes in RA dimensions were proportional to those observed for the RV, as demonstrated by greater RV middle and basal diameters observed in the athlete’s group, supporting the hypothesis that the modifications observed in athletes reflect the physiological change in hemodynamic loading conditions associated with intense training. In fact, as demonstrated by Doppler and TDI analysis, RV function does not deteriorate in the athlete’s heart despite the significant chamber dilatation. Conversely, athletes exhibit not only a normal RV systolic and diastolic function, but even a supernormal function, as demonstrated by higher values of E/A ratio and TAPSE observed in the present study in athletes as compared with normal subjects.
In the first pioneer studies concerning the athlete’s heart, mainly using chest percussion and X-rays, it was already documented that athlete’s heart is a symmetrical phenomenon regarding both left and right chambers [20, 21] and more recent echocardiographic studies have investigated the morphological changes of the RA and the RV induced by exercise training [6, 7, 22–24]. However, to date, no research has focused on RA function in athletes. A previous study by our research group demonstrated that 2D STE is a feasible technique for the assessment of RA longitudinal myocardial deformation in normal subjects, able to provide stimulating insights into the functional role of RA [9]. In the present study, we extended the previous observations providing new functional data about the role of RA in athlete’s heart, establishing for the first time the reference values of RA longitudinal strain in 100 top-level athletes obtained by 2D STE. This novel echocardiographic technique gives us the opportunity to go further the historical observations concerning the right heart, demonstrating that RA remodeling in top-level athletes is not limited to the morphology, but encompasses also a functional adaptation with a characteristic pattern of myocardial deformation dynamics. Two-dimensional STE analysis was able to detect these functional peculiarities, demonstrating that athletes exhibit significantly lower values of RA PALS and PACS as compared with controls.
A reduction of atrial strain values has been reported for the left atrium in association with cardiovascular disease, suggesting an impairment of left atrial performance [25]. However, we previously demonstrated that athletes show a peculiar reduction of left atrial global PALS and global PACS values in comparison with controls [8]. Moreover, a significant reduction of left atrial global PALS and global PACS has been also demonstrated in elite athletes during the regular season, with higher values observed at the beginning of the training program and significantly lower values found after 8 months of intensive exercise [26]. In this study, we observed that the decrease in RA strain values was associated with a significant increase in E/A ratio, a normal PALS/PACS ratio, and a normal RA stiffness parameter. These findings suggest that the reduction of left atrial strain values and, similarly, the reduction of RA PALS and PACS observed in athletes in the present study represent a physiological adaptation to intensive training and they should be interpreted as physiological phenomena rather than pathological abnormalities when evaluating RA function in highly trained athletes and can be included in the context of the so-called “athlete’s heart”.
We demonstrated that RA volume index and RA area are independent predictors of TPLS while RV E/A ratio and HR, together with RV basal diameter, are independent predictors of RA TPCS. Interestingly, while TPLS and TPCS were significantly differ between athletes and controls when expressed in absolute values, the difference was not significant when TPCS was indexed to RR, suggesting that resting bradycardia played a relevant influence on RA contraction strain parameters, reducing the RA contribution to RV filling by lengthening diastole. Thus, the present findings confirmed that top-level athletes show significant variations in HR occurred in close association with cardiac chamber enlargement. Athletes involved in sports with a high dynamic component are known to develop predominantly increase in chamber size, able to maintain, even in a condition of bradycardia, an adequate cardiac output at rest [1]. Moreover, the increase in chamber size is able to enhance stroke volume during exercise and, together with the increase in HR, is responsible for cardiac output augmentation. Thus, the variations in chamber size and in resting HR exhibited by athletes could have influenced the RA myocardial deformation dynamics, supporting the hypothesis of a global remodeling of the right heart. Furthermore, the significant difference in resting HR between athletes and controls could have influenced the analysis of RV diastolic function. In fact, training-induced bradycardia is able to prolong the diastolic filling period and to lengthen the diastasis during mid-diastole, increasing the E/A ratio and making complex the interpretation of diastolic function in athlete’s heart. Further researches are needed to confirm our findings and to clarify the determinants of the variations of RA PALS, PACS, TPLS, and TPCS values, associated with training and with HR changes.
The process of cardiovascular adaptation to exercise conditioning is associated in athletes not only to cardiac, but also to extracardiac modifications [6, 22, 27]. In this study, we reported that athletes show significantly higher values of inferior vena cava (IVC) diameter in comparison with controls, suggesting an extracardiac adaptation caused by prolonged training. In absence of cardiac diseases, the dilatation of inferior vena cava can be referred to a physiological adaptation to the augmented venous return, causing an increased volume load with an increased cardiac output. These results are in agreement with previous observations by Goldhammer et al. [22], demonstrating that IVC is dilated in highly trained athletes and suggesting that this phenomenon is related to the intensity of training and the number of training hours. The authors, through the collapsibility of the IVC, derived an index of RA pressure and assumed that RA pressure in athletes was somewhat higher than in control group, reporting a negative correlation between IVC diameter and collapsibility index, with a positive derived correlation between RA pressure and IVC diameter. Conversely, in the present study, to estimate RA pressure, we used the ratio between the tricuspid flow early diastolic velocity (E) and peak early diastolic velocity of the lateral tricuspid annulus (e′), the tricuspid annular E/e′ ratio, an echocardiographic index highly correlated to invasively measured RA pressure [11, 13]. Using this noninvasive parameter, we demonstrated for the first time that resting RA pressure did not substantially vary between athletes and controls. These findings suggest that the increase in IVC diameter, the great RA volume index, and the great RV diameters observed in elite athletes cannot be interpreted as markers of right diastolic impairment and do not likely represent a form of adaptation to pressure overload, but rather can be regarded as a physiologic remodeling related to the volume overload associated with intensive training.
Finally, in this study, the comparison among the three groups of athletes allowed us to demonstrate that sex- and sport-specific adaptations of athlete’s heart exist in subjects of different sex engaged in different sporting disciplines, providing further insights into a topic that is known to be somewhat controversial [28].
Limitations
Two-dimensional STE is a novel echocardiographic technique known to be dependent on frame rate as well as image resolution. Even if this technical limitation rarely represents a problem when evaluating athletes, RA myocardium is thinner than RV one as well as LA myocardium is thinner than left ventricular one: the thinness of the atria represents a possible technical limitation when evaluating the myocardial deformation by 2D STE. However, 2D STE is a semiautomatic technique and small manual adjustments are able to improve the tracking of RA endocardium in nearly all cases, allowing the assessment of RA longitudinal myocardial deformation dynamics with a good reproducibility [9]. Another limitation of this study is that the changes in RA morphology and function occurring with intensive training have been investigated at rest. It would be of interest to explore the myocardial deformation dynamics of right heart during the effort.
Conclusions
Top-level athletes exhibit greater RA area, volume, and volume index as compared with controls. The physiologic adaptation of RA to intensive training encompasses not only a morphological but also a functional remodeling, as demonstrated by a peculiar pattern of RA longitudinal myocardial deformation dynamics. We demonstrated that 2D STE is a useful tool to assess RA longitudinal myocardial deformation dynamics in the context of athlete’s heart.
References
Fagard R (2003) Athlete’s heart. Heart 89:1455–1461
Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE (2000) The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101:336–344
Naylor LH, George K, O’Driscoll G, Green DJ (2008) The athlete’s heart. A contemporary appraisal of the ‘Morganroth hypothesis’. Sports Med 38:69–90
Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU (2010) The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 11:81–96
Henriksen E, Landelius J, Wesslén L, Arnell H, Nyström-Rosander C, Kangro T, Jonason T, Rolf C, Lidell C, Hammarström E, Rinqqvist I, Friman G (1996) Echocardiographic right and left ventricular measurements in male elite endurance athletes. Eur Heart J 17:1121–1128
Erol MK, Karakelleoglu S (2002) Assessment of right heart function in the athlete’s heart. Heart Vessels 16:175–180
D’Andrea A, Riegler L, Golia E, Cocchia R, Scarafile R, Salerno G, Pezzullo E, Nunziata L, Citro R, Cuomo S, Caso P, Di Salvo G, Cittadini A, Russo MG, Calabrò R, Bossone E (2011) Range of right heart measurements in top-level athletes: the training impact. Int J Cardiol [Epub ahead of print]
D’Ascenzi F, Cameli M, Zacà V, Lisi M, Santoro A, Causarano A, Mondillo S (2011) Supernormal diastolic function and role of left atrial myocardial deformation analysis by 2D speckle tracking echocardiography in elite soccer players. Echocardiography 28:320–326
Padeletti M, Cameli M, Lisi M, Malandrino A, Mondillo S, Zacà V (2012) Reference values of right atrial longitudinal strain imaging by two-dimensional speckle tracking. Echocardiography 29:147–152
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate body surface area if height and weight be known. Arch Intern Med 17:863–871
Rudski LG, Lai WW, Afilao J, Hua L, Handsschumacher MD, Chandrasehkaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart W (2006) Recommendations for chamber quantification. Eur J Echocardiogr 7:79–108
Sade LE, Gulmez O, Eroglu S, Sezqin A, Muderrisoglu H (2007) Noninvasive estimation of right ventricular filling pressure by ratio of early tricuspid inflow to annular diastolic velocity in patients with and without recent cardiac surgery. J Am Soc Echocardiogr 20:982–988
Said K, Shehata A, Ashour Z, El-Tobqi S. Value of conventional and tissue Doppler echocardiography in the noninvasive measurement of right atrial pressure (2012) Echocardiography [Epub ahead of print]
Nageh MF, Kopelen HA, Zoghbi WA, Quinones MA, Nagueh SF (1999) Estimation of mean right atrial pressure using tissue Doppler imaging. Am J Cardiol 84:1448–1451
Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M (2009) Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 8(7):6
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Naqueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voiqt JU, Zamorano JL (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12:167–205
Kurt M, Wang J, Torre-Amione G, Nagueh SF (2009) Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2:10–15
Appleton CP, Kovács SJ (2009) The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2:6–9
Henschen S (1899) Skilanglauf und swiwettlauf: eine medizinische sport studie. Mitt med Klin Uppsala (Jena) 2:15–18
Keys A, Friedell HL (1938) Size and stroke of the heart in young men in relation to physical activity. Science 88:456–458
Goldhammer E, Mesnick N, Abinader EG, Sagiv M (1999) Dilated inferior vena cava: a common echocardiographic finding in highly trained elite athletes. J Am Soc Echocardiogr 12:988–993
Popovic D, Damjanovic S, Markovic V, Vujisic-Tesic B, Petrovic M, Nedeljkovic I, Arandjelovic A, Popovic B, Jakovljevic B, Stojiljkovic S, Ostojic SM (2011) Systolic right ventricular adaptive changes in athletes as predictors of the maximal functional capacity: a pulsed tissue Doppler study. J Sports Med Phys Fitness 51:452–461
Hauser AM, Dressendorfer RH, Vos M, Hashimoto T, Gordon S, Timmis GC (1985) Symmetric cardiac enlargement in highly trained endurance athletes: a two-dimensional echocardiographic study. Am Heart J 109:1038–1044
Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P (2011) Early detection of left atrial strain abnormalities by speckle tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 24:898–908
D’Ascenzi F, Cameli M, Lisi M, Zacà V, Natali B, Malandrino A, Benincasa S, Catanese S, Causarano A, Mondillo S (2012) Left atrial remodelling in competitive adolescent soccer players. Int J Sports Med 33:1–7 [ahead of print]. doi: 10.1055/s-0032-1304660
Zeppilli P, Vannicelli R, Santini C, Dello Russo A, Picani C, Palmieri V, Cameli S, Corsetti R, Pietrangeli L (1995) Echocardiographic size of conductance vessels in athletes and sedentary people. Int J Sports Med 16:38–44
Barbier J, Ville N, Kervio G, Walther G, Carré F (2006) Sports-specific features of athlete’s heart and their relation to echocardiographic parameters. Herz 31:531–543
Acknowledgments
The authors wish to thank Ferdinando Minucci, chairman of Mens Sana Basket Club, Massimo and Valentina Mezzaroma, chairman and vice-chairwoman of Siena Football Club, and Giancarlo Campinoti, chairman of Santa Croce Volleyball Club, all members of medical and coaching staff, athletes, and managers for their support in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Ascenzi, F., Cameli, M., Padeletti, M. et al. Characterization of right atrial function and dimension in top-level athletes: a speckle tracking study. Int J Cardiovasc Imaging 29, 87–94 (2013). https://doi.org/10.1007/s10554-012-0063-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0063-z