Abstract
We wanted to assess the performance of dual-source CT (DSCT) for evaluating the left atrial volume and function and we compared this performance to that of the standard reference technique, cardiac cine MR (CMR). Fifty-one patients who were referred for CT coronary angiography were included in the study. Two were subsequently excluded for having un-analyzable MR images at the onset of left atrial contraction. For the remaining 49 patients, the DSCT data sets and FIESTA (fast imaging employing steady-state acquisition) cines of the vertical long axis covering the left atrium and the short axis covering the left ventricle were obtained on the same day. All the images were analyzed to obtain the maximal left atrial volume (LAVmax), the minimal left atrial volume (LAVmin), the left atrial volume just before left atrium contraction (LAVp), the left atrial reservoir volume (LARV), the left atrial ejection fraction (LAEF), the left atrial passive emptying volume (LAPV), the left atrial active emptying volume (LAAV), the left atrial conduit volume (LACV), the left ventricular end-diastolic volume (LVEDV), the left ventricular end-systolic volume (LVESV) and the left ventricular stroke volume (LVSV) by using 3D semi-segmentation software and Report Card 2.0 software, respectively, for the DSCT and CMR. All the parameters were normalized to the body surface area. Intermodality agreement was tested through linear regression and Bland–Altman analyses. Repeated measurements were performed to determine the interobserver variability. The DSCT measurements resulted in good correlation (r > 0.75) compared with those of CMR. However, DSCT slightly, but insignificantly overestimated the indexed LAVmax, LAVmin, LAVp, LARV, LAPV, LAAV and LACV, as reflected by biases of 1.2, 0.9, 1.1, 0.3, 0.1, 0.2 and 0.4 ml/m2, respectively. The LAEF was minimally, but insignificantly underestimated as reflected by a bias of −0.6% (P = ns). The variability of the DSCT measurements was lower than that of the CMR values. DSCT provides highly reliable and reproducible measurements of the left atrial phasic volume and function in patients who are referred for coronary CT imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The left atrium (LA) serves multiple functions as it acts as a reservoir during ventricular systole, a conduit during early ventricular diastole and a booster pump during late ventricular diastole for blood returning from the lungs to the heart [1]. The principal function of the LA is to modulate left ventricular (LV) filling and the cardiovascular performance. It has been demonstrated that changes in the LA size and function are closely related to such cardiovascular diseases as diastolic dysfunction, cardiomyopathy, ischemic heart disease and valvular disease, and these changes act as barometers of cardiovascular outcomes [2–6]. The LA size and function are important factors in the development of atrial fibrillation and for determining the long term results of cardioversion. The LA size and function are risk factors for stroke and death [7–9]. It has been recently reported that the indexed LA volume is a more accurate measure of LA size and a more robust predictor of adverse events than the LA area or diameter [10]. Accurate and reproducible measurement of the LA volume and function is important for making the clinical diagnosis, risk stratification and guiding therapy during the care of patients with cardiac disease.
A newly developed dual-source computed tomography (DSCT) scanner equipped with two tubes and two corresponding detectors yields a heart rate independent temporal resolution of 83 ms [11]. In addition, the previous studies that have evaluated the ventricular function demonstrate that DSCT enables accurate assessment of the left ventricular function independent of the heart rate [12]. Because of the limited radiation dose, assessing the cardiac function is not the only purpose for a DSCT examination; rather, it is a by-product of coronary CT imaging.
There are currently no reports that have focused on assessing the LA phasic volume and function using DSCT and there are no reports that have compared DSCT and MRI for evaluating the left atrial function. Hence, the purpose of this study was to evaluate the performance of DSCT for determining the LA volume and function as compared to the reference standard, cine MRI.
Materials and methods
Study population
Between November 2007 and July 2008, those patients who were referred for a clinically indicated CT coronary angiography examination were screened for possible participation in this study. The referred patients with atrial fibrillation or other cardiac arrhythmias or cardiac valvular heart diseases and the patients with implanted pacemakers or defibrillators or with claustrophobia were excluded. Additionally, patients were excluded if they could not undergo a CT coronary angiography examination because of renal dysfunction (a serum creatinine levels higher than 1.5 mg/dl) or a previous allergic reaction to iodinated contrast media. A total of 51 patients (28 men and 23 women; mean age, 53 ± 13 years) met the study’s inclusion criteria. Of these, 45 had hypertension (10 of whom had coronary artery disease). All the others had normal hearts. Two patients were excluded from the analyses due to their un-analyzable MR images at the onset of left atrial contraction. For all the included patients, MRI was performed after CT on the same day. The subjects’ heart rates were continuously monitored throughout image acquisition by two modalities. No additional ß-blockers were administered in our study. Written informed consent was obtained from all the patients. The local ethics committee approved this study.
Dual-source CT imaging
DSCT was performed using a 64-slice DSCT scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany). The scanning range covered the entire heart from 1 cm below the level of the tracheal bifurcation to the diaphragm. A 50–75 ml bolus of iopamidol 370 (iopamidol injection; Bracco, Shanghai, China), followed by 20 ml of saline solution, was continuously injected into an antecubital vein at an injection rate of 4–5 ml/s. Injection was performed through an antecubital vein via a dual-head power injector (Stellant; Medrad, Indianola, USA). The CT angiography was triggered automatically and image acquisition started 4 s after the signal attenuation reached the predefined threshold of 100 HU at the aortic root. The data was acquired in a craniocaudal direction with a tube voltage of 100–135 kV and a tube current of 350–420 mAs. The other scan parameters were as follows: a slice collimation of 2 × 32 × 0.6 mm, a slice acquisition of 2 × 64 × 0.6 mm by means of a z-flying focal spot, a detector collimation of 64 × 0.5 mm thickness, a gantry rotation time of 330 ms, a pitch of 0.2–0.5 (depending on the heart rate), a reconstructed slice thickness of 0.75 mm (with an increment of 0.4 mm) and the use of a soft tissue convolution kernel (B26f). ECG-gated tube current modulation for reducing the radiation dose was used during scanning for all the patients, as was previously recommended [13]. Generally speaking, for the patients with a heart rate (HR) below 60 bpm, the range of the ECG-pulsing window with full tube current output was set from 60 to 80% of the R–R interval,it was set from 40 to 75% of the R–R interval for a HR 61–70 bpm, from 30 to 75% of the R–R interval for a HR 71–119 bpm and from 25 to 85% of the R–R interval for a HR above 120 bpm. The reduced percentage of the normal tube current outside the pulsing window was 80%. The estimated radiation dose in this study was about 6–10 mSv using this protocol. A monosegmental image reconstruction algorithm using data from both X-ray sources was used, and this resulted in a constant temporal resolution of 83 ms [11]. Retrospective ECG-gating was used to synchronize the data with the ECG, and 20 series of axial images were reconstructed every 5% of the R–R interval (0–95%) with an effective slice thickness of 0.75 mm, a reconstruction increment of 0.4 mm and a field of view of 180 × 170 mm (matrix: 512 × 512). The images obtained at the onset of the P-wave on ECG were also reconstructed using the same parameters. All the reconstructed DSCT data were post-processed on a dedicated workstation (Multi Modality Work Place, CT 2008A, Siemens).
Cine MR imaging
All the CMR examinations were performed with the patient in the supine position on a 3.0 T MR scanner (Signa Excite HD 3T, General Electric Medical Systems) with an eight-channel cardiac coil. The CMR images were acquired with retrospective ECG R-wave gating. After localizing the images, piloting was performed in the vertical long axis, the horizontal long axis and the short-axis planes using real-time sequence. FIESTA cines of the vertical long axis, the horizontal long axis and the short-axis planes were then obtained. The parameters for the FIESTA cines were a TR of 3.7 ms, a TE of 1.6 ms, a flip angle of 45°, a field of view of 350 × 350 mm, a pFOV of 1.0 and a matrix size of 200 × 224 with a 125 Hz/pixel bandwidth, a vps of 16, a ETL of 1, a NEX of 1.00 and a phase of 20.
The atrial slices were planned to be parallel to the line through the tip of the mitral valve and the apex on the horizontal long axis cine at ventricular end-diastole (0 ms) and then the atrial images were generated in the vertical long axis. The vertical long axis was chosen because Jarvinen et al. [14] showed that the definition of the atrioventricular borders (the atrioventricular valve annulus) is optimal in this projection. The left atrium was covered by 7–14 scans with a 5-mm slice thickness. The short axis stack was obtained parallel to the atrioventricular groove and covering the entire left ventricle by 10–15 scans with each having an 8-mm slice thickness. All the images were acquired during 10–15 s breath-holds at end-inspiration.
Image analysis
The LAVmin occurs at mitral valve closure, and the LAVmax occurs just before mitral valve opening. The LAVp occurs at the onset of the P-wave on ECG. The LA volumes and functions were defined as follows [15]:
-
Left atrial passive emptying volume (LAPV) = LAVmax − LAVp
-
Left atrial active emptying volume (LAAV) = LAVp − LAVmin
-
Left atrial conduit volume (LACV) = LVSV − LASV = (LVEDV − LVESV) − (LAVmax − LAVmin)
-
Left atrial reservoir volume (LARV) = LAVmax − LAVmin
-
LAEF = (LAVmax − LAVmin)/LAVmax
DSCT
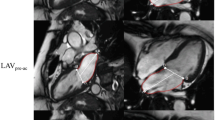
All the DSCT data were transferred to a dedicated workstation (Multi Modality Work Place, CT 2008A, Siemens) for further analysis. Two independent operators with more than 5 years experience in cardiovascular imaging post-processed the data. Assessment of the LA or LV volumes was performed with a dedicated software package for cardiac function analysis (Circulation) with threshold-based region-growing 3D segmentation of the LA or LV cavity. When necessary, the LV or LA endocardial contours were corrected visually for accuracy on all the CT images that had a discernible cardiac cavity, and this was done frame by frame in 360° rotational MPRs (Fig. 1). The papillary muscles and trabeculae were included in the LV cavity for the delineation of the LV endocardial border. The atrial appendages and pulmonary veins were excluded from the delineation of the LA endocardial border. The post-processing software automatically calculated the LV or LA volumetric parameters. All the volumes were normalized to the body surface area (BSA).
The threshold-based region-growing 3D segmentation technique in DSCT provided true 3D rendering of the maximal LA (left atrial) volume without having to make any geometrical assumptions. Images a and b show the 2D graphics for defining the LA boundaries. Images c and d show the corresponding 3D graphics for rendering the LA volume
CMR
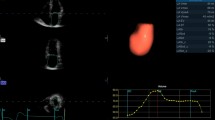
All the CMR image data were analyzed offline, using commercial software for the analysis of the CMR images (Report Card 2.0, General Electric Medical Systems), by another two independent operators who each had more than 5 years experience in cardiovascular imaging. The left ventricular and left atrial endocardial borders were traced manually in each phase of the cardiac cycle on each slice of the multislice acquisitions (Fig. 2).
All the functional parameters from the CMR were calculated according to the modified Simpson’s method. The end-diastolic and end-systolic phases were visually determined as the phases with the largest and smallest ventricular cavities, respectively. In this study, the papillary muscles were included in the LV volumes, and the atrial appendages and pulmonary veins were excluded from the atrial volumes. The left atrial volume cycle and its measurements are shown schematically in Fig. 3. The LAVp can be read off as point B from the volume-time curves. In the majority of cases, point B was the second of two peaks that corresponded to active atrial systole after passive atrial emptying. In two patients, the atrial volume-time curves yielded only one peak, and this precluded evaluating the volume at the onset of atrial contraction and the subsequent functional calculations for these patients. All the volumes were normalized to the body surface area (BSA).
Statistical analysis
All the continuous variables were expressed as means ± standard deviations (SD). The correlation between the DSCT and CMR was assessed using linear regression analysis with Pearson’s correlation coefficients. The degree of agreement between the DSCT and CMR reference values was evaluated according to the method of Bland and Altman [16] by calculating the bias (mean difference) and the 95% limits of agreement (two SDs around the mean difference). A one-sample t test with a 2-sided alternative was used to determine whether the resulting mean difference was significant from zero, and this represented a significant under or overestimation with using DSCT.
Pearson correlation coefficients were calculated to evaluate the interobserver agreement between the two observers for all the parameters. Interobserver variability was assessed by the Bland–Altman method to compare differences of the observations from the mean of the observations [16]. For all statistical analyses, a P-value of less than 0.05 was considered significant. Data analysis was performed using commercially available software (SPSS 13.0, Chicago, IL, USA).
Results
The mean heart rate during the DSCT evaluations was 69 ± 14 beats/min (median = 69 beats/min; range = 47–113 beats/min). The mean heart rate during the CMR scanning was 67 ± 11 beats/min (median = 67 beats/min; range = 50–110 beats/min) with no significant difference between the modalities (P = ns). The mean BSA was 1.81 ± 0.21 m2 (range = 1.45–2.33).
The results of DSCT measurements of the LA volume indexed to the BSA were: LAVmax = 47.4 ± 11.1 ml/m2, LAVmin = 22.2 ± 6.9 ml/m2 and LAVp = 34.8 ± 8.8 ml/m2. The LA volume indexed to the BSA measured by CMR was: LAVmax = 46.2 ± 9.7 ml/m2, LAVmin = 21.3 ± 5.8 ml/m2 and LAVp = 33.6 ± 7.8 ml/m2.
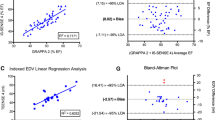
Figure 4 shows the results of the linear regression analysis for the DSCT and CMR measurements of the indexed LA volume and function. All the volumetric and functional measurements obtained with DSCT were significantly correlated with the data obtained with CMR at the P < 0.001 level. The correlation coefficients were: 0.89 for the indexed LAVmax, 0.90 for the indexed LAVmin, 0.90 for the indexed LAVp, 0.80 for the indexed LARV, 0.78 for the indexed LAEF, 0.82 for the indexed LAPV, 0.80 for the indexed LAAV and 0.76 for the indexed LACV.
The scatter plots and linear correlation analysis of the left atrial (LA) volumes and functions between DSCT and CMR. a The maximal LA volumes. b The minimal LA volumes. c The LA volumes just before LA contraction. d The LA reservoir volumes. e The LA ejection fraction. f The LA passive emptying volumes. g The LA active emptying volumes. h The LA conduit volumes
Figure 5 summarizes the results of the Bland–Altman analysis for the DSCT and CMR measurements of the indexed LA volume and function. Compared to the CMR, the Bland–Altman analysis revealed a mean bias for the indexed LAVmax of 1.2 ml/m2, that for the indexed LAVmin was 0.9 ml/m2, that for the indexed LAVp was 1.1 ml/m2, that for the indexed LARV was 0.3 ml/m2, that for the indexed LAPV was 0.1 ml/m2, that for the indexed LAAV was 0.2 ml/m2 and that for the indexed LACV was 0.4 ml/m2, respectively. The LAEF was slightly, but insignificantly underestimated, as reflected by a bias of −0.6%. No significant differences of the mean difference from zero were found for all the LA volumetric and functional parameters (P = ns).
The Bland–Altman plots of agreement between the DSCT and CMR measurements of the left atrial (LA) volumes and functions. a The maximal LA volumes. b The minimal LA volumes. c The LA volumes just before LA contraction. d The LA reservoir volumes. e The LA ejection fraction. f The LA passive emptying volumes. g The LA active emptying volumes. h The LA conduit volumes
Table 1 shows the interobserver agreement for the DSCT and CMR measurements of the indexed LA volume and function. The linear regression coefficients (r) ranged from 0.86 to 0.99 for DSCT, and they ranged from 0.85 to 0.98 for CMR, indicating an excellent correlation between the two readers. Bland–Altman analysis for assessing the degree of agreement between the two readers revealed minimal mean differences and all the measurements were within close limits of agreement for all the parameters.
Discussion
To the best of our knowledge, this is the first report that has focused on assessing the left atrial phasic function using DSCT. In a previous study by Christiaens et al. [17], the LAVmax, LAVmin and LAEF were measured using 64-MSCT, but the other left atrial functional variables were not evaluated. The left atrial function is impaired in a number of cardiovascular outcomes [2–5, 18–20]. With abnormal LV relaxation, the relative contribution of the LA reservoir and the LA contractile function increases, and the conduit function decreases. However, as the LV filling pressure progressively increases with advancing diastolic dysfunction, the LA predominantly serves as a conduit [6]. Thus, comprehensive evaluation of the left atrial function is important, and the LA size might be an important clinical factor to stratify the risk of preclinical cardiovascular disease.
Retrospective ECG-gated DSCT scanning makes any phase throughout the cardiac cycle easily available for assessment, and especially for reconstruction at the onset of the P-wave on ECG. In the current study, when the corresponding phases were reconstructed, the maximal and minimal LA volumes and the LA volume before left atrial contraction could be measured, and several LA functional parameters were calculated. We used a new DSCT scanner with an increased temporal resolution of 83 ms to assess the left atrial volume and function during the whole of the cardiac cycle. Our primary findings demonstrated the feasibility and reproducibility of DSCT for detecting the indexed left atrial phasic volume and function changes. The close linear relationship between DSCT and CMR for the measurement of the indexed LA volume and function was confirmed by our results, as was reflected by the high correlation coefficients obtained for all the measured indices .
Interestingly, several studies have demonstrated systematic and consistent overestimation of the LV volume using MDCT when compared to using MRI [21, 22]. Similarly in our study, although no significant differences of the mean difference from zero were found for all the LA volumetric and functional measurements, there was a trend by DSCT towards overestimating the indexed LA volumes as compared with the CMR values, and this resulted in a small trend towards underestimating the LAEF, as reflected by a negative bias. There was also a trend by DSCT towards overestimating the LA reservoir, contractile and conduit functions, as compared with the CMR values.
Several possibilities can cause these tendencies. First, imaging of the moving heart requires high temporal resolution to achieve an artifact-free display of myocardial contraction over the cardiac cycle [23]. High temporal resolution is needed to identify the proper image reconstruction windows for accurately measuring the LA volume and function. Compared to CMR, which has a temporal resolution of 32 ms [24], DSCT technology provides a temporal resolution of 83 ms, and this is independent of heart rate. Because of the relatively low temporal resolution of the reformatted data, which is typically reconstructed at 20 phases of the cardiac cycle, the LA volume values measured by DSCT are subject to overestimation. CMR, with its relatively high temporal resolution, can easily catch the exact phase of the LA throughout the whole cardiac cycle. Second, an increase of image noise during the systolic phase that is due to tube current modulation may contribute to the overestimation of the LA volume. Yet ECG-gated tube-current modulation with using DSCT did not hamper reliably assessing the global and regional LV function in a previous animal study [25]. In our study, tube current modulation did not prevent delineating the endocardial border or LA segmentation in the systolic phase. Third, bolus injection of iodine contrast solution with a high speed and large volume could have resulted in hemodynamically transient changes with preload and negative inotropic effects. Finally, additional medicines such as ß-blockers could result in a change of the LA volume and so this could hinder direct comparisons between DSCT and CMR when ß-blockers are not used during these imaging examinations. ß-blockers were not used in this study to avoid this effect.
The DSCT measurements were found to be more reproducible than that of CMR, as was reflected by the minimal interobserver variability of all the measured indices. A plausible explanation for this finding would be that the spatial resolution of DSCT is higher than that of CMR, and the threshold-based region-growing 3D segmentation technique in DSCT provides true 3D rendering of the LA volume without having to make any geometrical assumptions. In contrast, the slice thickness of CMR for the acquisition of the LA images is 5 mm, and a small difference in tracing the left atrial endocardial contours can cause a large effect on the left atrial volume value with using the modified Simpson method. In addition, the interobserver agreement of these left atrial measurements was not as high as that of the left ventricle measurements, illustrating the difficulty in drawing the irregular left atrial contours, and particularly when excluding the left atrial appendage and the pulmonary veins. The difficulty in defining the boundary between the pulmonary veins and the left atrial body, or between the left atrial appendage and the left atrial body causes measurement variability. However, the left atrial measurement variability was acceptable in this study.
Traditionally, LA size has been studied with performing 2-dimensional (2D) echocardiography [26]. However, the cyclic changes of the LA volume can not be observed directly by these methods because the shape of the LA changes during the heart cycle, and the pattern is altered if the loading conditions change [27]. Therefore, these techniques present only a snapshot view of the diastolic function. In contrast, cardiac MRI has been proved to be an effective alternative for accurately assessing the LA volume and the phasic volumetric function of the LA during the heart cycle [28, 29]. However, the cost and limited availability of MRI mean that this technique is largely impractical for routine clinical use. DSCT provides combined morphologic and functional clinical data on the patients who undergo CT coronary angiography with using one dataset.
Several limitations of this study should be mentioned. First, the vertical long axis was chosen to obtain the LA cine MR images, yet short axis or horizontal orientation acquisition is also feasible. Similarly to Jarvinen et al. [14], in our experience, it is optimal to define the atrioventricular borders in the vertical long axis. Second, the LA volume and function postprocessing is time-consuming. But for an experienced observer, the required time is about 10 min. Therefore, with the improved post-processing techniques and increased experience, the post-processing time is not a problem. Third, we did not evaluate the intraobserver variability of both modalities.
In summary, with its increased temporal resolution, DSCT allows for comprehensive assessment of the heart and the information on the coronary vessels and cardiac function can be conveniently obtained from a single dataset without any additional radiation exposure or cost.
References
Barbier P, Solomon SB, Schiller NB et al (1999) Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100:427–436
Rossi A, Cicoira M, Zanolla L et al (2002) Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol 40(8):1425
Giannuzzi P, Temporelli PL, Bosimini E et al (1996) Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol 28(2):383–390
Moller JE, Hillis GS, Oh JK et al (2003) Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 107(17):2207–2212
Beinart R, Boyko V, Schwammenthal E et al (2004) Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol 44(2):327–334
Prioli A, Marino P, Lanzoni L et al (1998) Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol 82(6):756–761
Benjamin EJ, D’Agostino RB, Belanger AJ et al (1995) Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation 92(4):835–841
Sanfilippo AJ, Abascal VM, Sheehan M et al (1990) Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82(3):792–797
Vaziri SM, Larson MG, Benjamin EJ et al (1994) Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham heart study. Circulation 89(2):724–730
Tsang TS, Abhayaratna WP, Barnes ME et al (2006) Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 47(5):1018–1023
Flohr TG, McCollough CH, Bruder H et al (2006) First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 16(2):256–268
Brodoefel H, Kramer U, Reimann A et al (2007) Dual-source CT with improved temporal resolution in assessment of left ventricular function: a pilot study. AJR Am J Roentgenol 189(5):1064–1070
Leschka S, Scheffel H, Desbiolles L et al (2007) Image quality and reconstruction intervals of dual-source CT coronary angiography: recommendations for ECG-pulsing windowing. Invest Radiol 42(8):543–549
Jarvinen V (1996) Assessment of left and right atrial function and transmitral flow profiles by magnetic resonance imaging (thesis). University of Finland, Helsinki
Thomas L (2007) Assessment of atrial function. Heart Lung Circ 16(3):234–242
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Christiaens L, Lequeux B, Ardilouze P et al (2009) A new method for measurement of left atrial volumes using 64-slice spiral computed tomography: comparison with two-dimensional echocardiographic techniques. Int J Cardiol 131(2):217–224
Cabin HS, Clubb KS, Hall C et al (1990) Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol 65(16):1112–1116
Gardin JM, McClelland R, Kitzman D et al (2001) M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the cardiovascular health study). Am J Cardiol 87(9):1051–1057
Pritchett AM, Mahoney DW, Jacobsen SJ et al (2005) Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 45(1):87–92
Stolzmann P, Scheffel H, Schertler T et al (2008) Radiation dose estimates indual-source computed tomography coronary angiography. Eur Radiol 18(3):592–599
van der Vleuten PA, Willems TP, Götte MJ et al (2006) Quantification of global left ventricular function: comparison of multidetector computed tomography and magnetic resonance imaging. A meta-analysis and review of the current literature. Acta Radiol 47(10):1049–1057
Savino G, Zwerner P, Herzog C et al (2007) CT of cardiac function. J Thorac Imaging 22(1):86–100
Juergens KU, Grude M, Maintz D et al (2004) Multi-detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology 230(2):403–410
Mahnken AH, Bruners P, Schmidt B et al (2009) Left ventricular function can reliably be assessed from dual-source CT using ECG-gated tube current modulation. Invest Radiol 44(7):384–389
Ujino K, Barnes ME, Cha SS et al (2006) Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol 98(9):1185–1188
Abhayaratna WP, Seward JB, Appleton CP et al (2006) Left atrial size physiologic determinants and clinical applications. J Am Coll Cardiol 47(12):2357–2363
Jarvinen VM, Kupari MM, Poutanen VP et al (1996) Right and left atrial phasic volumetric function in mildly symptomatic dilated and hypertrophic cardiomyopathy: cine MR imaging assessment. Radiology 198(2):487–495
Hudsmith LE, Petersen SE, Francis JM et al (2005) Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 7(5):775–782
Acknowledgments
The authors appreciate the technical assistance of Yike Zhao, Miao Guo and Zixu Yan. We also want to thank Hong Jiang, Honghong Tie, Haixia Yang and Jinli Xiao for their aid in recruiting the patients for the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, Z., Zhang, Z., Yu, W. et al. Assessing the left atrial phasic volume and function with dual-source CT: comparison with 3T MRI. Int J Cardiovasc Imaging 26 (Suppl 1), 83–92 (2010). https://doi.org/10.1007/s10554-009-9569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9569-4