Abstract
Purpose This study aims to identify the clinical implications of myocardial perfusion defects after chemoradiation therapy (CRT) in patients with esophageal and lung cancer. Methods We retrospectively compared myocardial perfusion imaging (MPI) results before and after CRT in 16 patients with esophageal cancer and 24 patients with lung cancer. New MPI defects in the radiation therapy (RT) fields were considered related to RT. Follow-up to evaluate for cardiac complications and their relation with the results of MPI was performed. Statistical analysis identified predictors of cardiac morbidities. Results Eleven females and twenty nine males at a mean age of 66.7 years were included. Five patients (31%) with esophageal cancer and seven patients (29%) with lung cancer developed myocardial ischemia in the RT field at mean intervals of 7.0 and 8.4 months after RT. The patients were followed-up for mean intervals of 15 and 23 months in the esophageal and lung cancer groups, respectively. Seven patients in each of the esophageal (44%) and lung (29%) cancer patients (P = 0.5) developed cardiac complications of which one patient with esophageal cancer died of complete heart block. Six out of the fourteen patients (43%) with cardiac complication had new ischemia on MPI after CRT of which only one developed angina. The remaining eight patients with cardiac complications had normal MPI results. MPI result was not a statistically significant predictor of future cardiac complications after CRT. A history of congestive heart failure (CHF) (P = 0.003) or arrhythmia (P = 0.003) is a significant predictor of cardiac morbidity after CRT in univariate analysis but marginal predictors when multivariate analysis was performed (P = 0.06 and 0.06 for CHF and arrhythmia, respectively). Conclusions Cardiac complications after CRT are more common in esophageal than lung cancer patients but the difference is not statistically significant. MPI abnormalities are frequently seen after CRT but are not predictive of future cardiac complications. A history of arrhythmia or CHF is significantly associated with cardiac complications after CRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiation therapy (RT) is associated with multiple cardiac complications when the heart is included in the RT field. These complications have clinical implications and may include acute and chronic pericarditis, coronary artery disease (CAD), conduction abnormalities, valvular insufficiency, and cardiomyopathy [1]. Old RT techniques used to treat the breast/chest wall and draining lymph nodes in breast cancer patients resulted in delivery of relatively high doses to a substantial volume of the heart. There is convincing evidence of excess cardiovascular morbidity and mortality in patients treated with these techniques [2, 3]. These old RT techniques used orthovoltage radiation, large fraction size, deep tangential Cobalt-60 fields and/or direct anterior internal mammary lymph node irradiation. Also, the reported long-term relative risk of death due to a fatal myocardial infarction in patients with lymphoma treated with mediastinal RT techniques is increased from 1.5 to 3.0 times that of unirradiated patients [4, 5]. There is conflicting data in patients treated with modern techniques regarding the impact of radiation on cardiovascular morbidity and mortality [6–8]. Harris and colleagues have recently demonstrated an overall difference in mortality from cardiac causes in left-sided versus right-sided breast cancer patients using contemporary tangential beam techniques [9]. This group reported a higher rate of cardiac deaths during the second decade post-treatment in left-sided breast cancer patients treated with RT of 6.4% compared with 3.5% in right sided breast cancer patients. On the other hand Giordano et al. found no difference in cardiac morbidity in left sided versus right sided breast cancer patients treated with new RT techniques [7]. Multiple studies have detected myocardial perfusion abnormalities in the heart corresponding with the RT field in left sided breast cancer patients even with modern RT techniques [10–12]. Some of these defects developed as early as 6 months after RT. The perfusion defects were also found to be associated with an increased incidence of chest pain [13]. Another recent investigation by Heidenreich and colleagues showed a high prevalence of stress-induced signs of ischemia and significant CAD in patients who received at least 35 Gy to the mediastinum for Hodgkin Disease at a young age [14].
Long-term effects of RT on the heart have been mainly described in breast cancer and lymphoma patients who tend to have long survival after therapy. On the other hand, short-term cardiac effects of RT in patients with tumors located closer to the heart, such as lung and esophageal tumors is not well investigated, despite the fact that this group of patients constitutes a large percentage of cancer patients who receive RT. Additionally, these patients usually receive high radiation doses to a significant portion of the heart due to the close proximity of these tumors to the heart. It is generally perceived that esophageal cancer and lung cancer patients do not survive long enough to manifest the cardiac morbidity or complications secondary to RT or chemoradiation therapy (CRT). However, the improvements in anticancer therapies have resulted in longer survival of cancer patients than previously anticipated to manifest the co-morbidities of these therapies [15]. For example, lung cancer is one of the cancers for which the 5-year survival rate has improved over the past decade [16]. Thus, some cancer patients may outlive their cancers to die of cardiac causes. Thus, the knowledge of the clinical impact of radiation induced myocardial perfusion defects would guide towards improved monitoring and management of esophageal and lung cancer patients who receive CRT.
This study aimed at investigating the clinical implications of myocardial perfusion abnormalities detected using myocardial perfusion imaging (MPI) in esophageal and lung cancer patients after CRT as well as the incidence of cardiac morbidity and mortality in these patients. Additionally, we attempted to identify predictors of cardiac morbidity after CRT.
Methods
After obtaining the approval of the institutional review board, a retrospective review of a database containing prospectively collected results of MPI studies in the Department of Nuclear Medicine was performed for the interval from March 2003 until October 2006. Patients with lung cancer and esophageal cancer who underwent MPI studies before and after CRT were included in the study. Patients with abnormal MPI results on a baseline MPI study prior to CRT were excluded from the study. Additionally, patients who underwent repeat MPI studies at an interval less than 2 months after the completion of RT were also excluded from the study since myocardial perfusion abnormalities seen in this early interval might be related to early transient inflammation and edema from the RT. The patients’ demographics and data regarding tumor type, disease stage, CRT, risk factors for CAD, history of arrhythmia and/or congestive heart failure (CHF) and MPI results were collected. Clinical course and outcome with regard to cardiac morbidity and/or mortality were assessed in all patients. Cardiac morbidity included patients who developed new clinical symptoms of cardiac etiology after the completion of CRT or patients who experienced an exacerbation or aggravation of a previously treated and stable cardiac condition. Cardiac mortality was defined as death related to a cardiac cause. Comparison between the incidence of cardiac morbidity and mortality in patients who developed new MPI defects in the RT field after CRT and those who did not was performed. Additionally, statistical analysis of multiple factors was performed to identify possible predictors of cardiac morbidity after CRT in both esophageal and lung cancer patients.
MPI procedure and interpretation
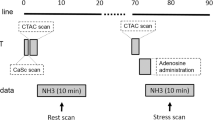
The patients were instructed to discontinue taking any calcium channel blockers or beta-blockers and avoid consuming caffeine for 24 h prior to undergoing stress tests. All patients underwent a single-day dual-isotope protocol. On the day of the MPI, each patient was injected with 111 MBq of thallium-201 chloride at rest. Single photon emission computed tomography (SPECT) images of the heart were acquired 10 min later using a dual-head gamma camera. Subsequently, the patients were stressed either pharmacologically or with exercise and were injected intravenously with 925–1,110 MBq of technetium-99m tetrofosmin at peak stress. This was followed by a 20–30 min rest period, during which the patient was allowed to drink juice and eat crackers. Stress gated SPECT images were subsequently obtained. The rest and stress SPECT acquisition parameters were: 180° arc, 64 views, 25 second/view, 64 × 64 matrix, and 1.45 zoom, with eight intervals for gated stress acquisition. The raw SPECT data was processed using filtered back-projection reconstruction with a Butterworth filter, order of five, and a cut-off frequency of 0.5 Nyquist (0.76 cycles/cm).
The MPI studies are routinely interpreted by one out of five routine readers on a daily bases and were independently reviewed by an additional expert reader who was blinded to the patients’ clinical information and the RT planning field or dose. Differences in interpretations between the routine reader and the blinded expert reader occurred in only three studies, in which the expert reader interpretation was used for the data analysis. Perfusion abnormalities were qualitatively graded as scar; ischemia; or mixed ischemia with scar. Functional information regarding left ventricular ejection fraction (LVEF) was also collected. None of the patients had significant arrhythmia during the acquisition of the functional information that would affect the accuracy of the LVEF results.
Statistical analysis
Fisher’s exact test was used to compare proportions between groups. Logistic regression analysis was used to assess the relationship between cardiac complications and covariates of interest, including sex, age, RT technique, mean heart radiation dose, volume of heart irradiated, V30 (percent volume of heart receiving 30 Gy), V40 (percent volume of heart receiving 40 Gy), V50 (percent volume of heart receiving 50 Gy), risk factors for CAD, history of CHF and arrhythmia. Odds ratios and corresponding 95% confidence intervals were estimated from these models. All parameters were tested in univariate analysis, and then factors that were significantly associated with complications were jointly modeled in a multivariate model. Additionally, logistic regression analysis to compare the frequency of risk factors for CAD in esophageal versus lung cancer patients was performed. All statistical tests were two-sided, and P-values of 0.05 or smaller were considered to be statistically significant. Statistical analysis was carried out using the SAS software program version 9 (SAS Institute, Cary, NC).
Results
The patients tumor types and stages of disease in both esophageal and lung cancer patient groups as well as RT techniques are summarized in Table 1. The prevalence of risk factors for CAD were comparable in esophageal versus lung cancer patients except for family history of CAD which was more prevalent in lung cancer patients (44% in lung cancer patients versus 6% in esophageal cancer patients, P = 0.007).
Esophageal cancer group
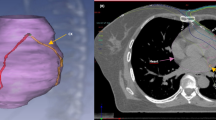
The esophageal cancer group consisted of 2 female and 14 male patients with a mean age of 67.6 years (range 48–85 years). Repeat MPI studies were performed after completion of RT at a mean interval of 7.0 months (median = 5.3 months) in this group of patients. The total radiation dose in 14/16 patients was 50.4 Gy, one patient received a total dose of 30.0 Gy and another patient received a total dose of 51.2 Gy. The mean heart radiation dose was 30.0 Gy (median = 30.0 Gy) and the mean heart volume irradiated was 734.7 cc (median = 685.8 cc). The mean V30 (mean heart volume receiving >30 Gy), V40 (mean heart volume receiving >40 Gy), and V50 (mean heart volume receiving >50 Gy) were 51, 31, and 16%; respectively. All patients in the esophageal cancer group received concurrent chemotherapy with RT. Five patients (31%) in the esophageal cancer group developed ischemia in the inferior segments which were in the RT field. Additionally, one patient developed anteroseptal ischemia after CRT which was not included in the RT field. The patients in the esophageal cancer group were followed for a mean interval of 14.6 months (median = 10.9 months, range = 2.8–34.8 months). During the follow-up interval seven patients (44%) developed cardiac complications (Table 2). One of these patients died (6%) of complete heart block and CHF at 12 months after CRT. This patient had no significant known cardiac disorders prior to CRT. Three of the patients who developed cardiac morbidities after CRT demonstrated ischemic changes on repeat MPI after CRT, and one of them was the patient who died of cardiac complications (Fig. 1). The remaining three patients who developed new MPI abnormalities after CRT did not suffer from cardiac morbidities. Thus, new MPI abnormalities after CRT were not predictive of future cardiac morbidities (P = 1.00). At the end of the follow-up period, 11/16 patients (69%) were deceased, 10 of whom died of noncardiac causes.
Short axis, vertical long axis and horizontal long axis slices of a stress MPI study before (a) and after CRT (b) demonstrating new ischemia (arrows) in a patient with esophageal cancer who died of complete heart block and worsening CHF 12.0 month after CRT. c Sagittal and coronal slices of the simulation CT with the RT plan demonstrating correlation of the irradiated part of the heart with the area of myocardial ischemia in (b)
There were no significant changes in functional results between the MPI studies performed before and after CRT in this group of patients. The mean LVEF for the baseline MPI studies was 63.2 ± 7.8% prior to CRT and 62.8 ± 10.7% after CRT (P = 0.78).
Logistic regression analysis of multiple factors that may predict higher risk of cardiac complications after CRT showed arrhythmia or a history of prior arrhythmia to be significantly associated with the incidence of cardiac complications after CRT (add odds ratio and 95% CI, P = 0.019). A history of CHF was weakly associated with cardiac morbidity after CRT (add OR, 95% CI, P = 0.06). No multivariate analysis was performed for this group because only one factor, which is arrhythmia, was significantly associated with complications.
Lung cancer group
The lung cancer group consisted of 9 female and 16 male patients with a mean age of 66.2 years (range 49–81 years). The mean total radiation dose, mean heart radiation dose and mean heart volume irradiated were 57.8 Gy (median = 61.5 Gy), 15.5 Gy (median = 16.1 Gy), and 645.8 cc (median = 621.5 cc), respectively. Additionally, the mean V30, V40, and V50 were 22, 16, and 9%; respectively. Twenty two out of twenty four patients in lung cancer group received concurrent or adjuvant chemotherapy with the RT and two received RT alone. Repeat MPI studies were performed after completion of RT at a mean interval of 8.4 months (median = 6.3 months) in this group. Anterior/septal new ischemia in seven patients (29%) in lung cancer group developed after CRT and these segments were included in the RT field. The patients were followed-up for a mean interval of 23.1 months (median = 14.6 months, range = 2.3–53.2 months) with regard to their cardiac morbidities or mortalities. Seven patients (29%) developed cardiac complications and morbidities after CRT but none of the patients died of cardiac cause (Table 3). Three of these patients had new ischemia on repeat MPI after CRT and the remaining four patients had normal MPI results. On the other hand, four additional patients developed new ischemic changes as per the MPI results after CRT but did not develop any cardiac complications or morbidities (P = 0.37). Six patients (25%) were deceased at the end of the follow-up period, all of them of noncardiac causes.
Similar to the esophageal cancer group, we observed no significant changes in functional results of the MPI studies as reflected in a mean LVEF prior to CRT of 63.4 ± 9.5% and after CRT of 63.5 ± 10.3% (P = 0.95).
History of arrhythmia or the presence of arrhythmia prior to CRT was also significantly associated with higher risk of cardiac complications after CRT (OR: 0.12, 95% CI: (0.02–0.90), P = 0.039). History of CHF also had a trend to be associated with higher incidence of cardiac complications but again did not reach statistical significance (P = 0.08) as is the case in the esophageal cancer group. Thus, multivariate analysis was also not performed in lung cancer patients.
All patients combined
When the total study population including both esophageal and lung cancer patients were considered in univariate logistic regression analysis, arrhythmia (P = 0.003) and CHF (P = 0.003) were significantly associated with future cardiac complications after CRT. The remaining tested variables did not reach statistical significance as predictors of future cardiac complications in the total study population. When arrhythmia and CHF were included in a multivariate analysis, both CHF (OR = 0.13, 95%CI: (0–1.14), P = 0.065) and arrhythmia (OR: 0.06, 95% CI: (0.02–1.07), P = 0.060) demonstrated marginal statistical significance, as predictors of future cardiac complications.
Discussion
In this study we investigated the incidence of cardiac morbidity and mortality and their relation to abnormal MPI studies after CRT in esophageal and lung cancer patients. The incidence of clinically manifested cardiac complications after CRT was 44% and 29% (P = 0.50) in patients with esophageal cancer and lung cancer, respectively. There was one death (6%) related to complete heart block in the esophageal cancer group after CRT but no cardiac related mortality in the lung cancer group. RT factors including RT technique, mean heart dose and volume as well as cardiac V30, V40 and V50 did not demonstrate significant association with the incidence of future cardiac complications. However, patients with underlying or prior conduction abnormalities and/or CHF are more likely to develop cardiac complications after CRT. Thus, further efforts and attempts at more targeted RT e.g. proton therapy [17, 18] or the use of cardioprotective agents [19] is warranted in such patient population.
We found that 33% of the lung cancer patients and 43% of the esophageal cancer patients who had cardiac morbidities demonstrated abnormal MPI studies after CRT. However, only one patient in the esophageal cancer group experienced anginal chest pain 7.5 months after CRT and another patient died of complete heart block. Findings of ischemia after CRT using MPI should alert clinicians for prompt therapy and closer monitoring of these patients. On the other hand, many of the cardiac morbidities after CRT were not associated with abnormalities on repeat MPI. These morbidities were mostly related to new onset arrhythmias, exacerbation of a prior stable arrhythmia or new pericardial effusions after CRT. Thus, MPI findings after CRT were not predictive of future cardiac complications (P = 1.0 and 0.37 in esophageal and lung cancer patients, respectively).
Interestingly, this study has also confirmed our previously identified specific patterns of MPI abnormality of inferior wall ischemia noted in distal esophageal cancer patients [20] and anterior/septal ischemia pattern in centrally located lung cancer patients [21]. This inferior ischemia in esophageal cancer patients is usually due to the proximity of the more common distal esophageal tumors to the heart resulting in inclusion of the inferior wall of the left ventricle in the RT field. On the other hand central lung tumors are usually in close proximity to the anterior wall of the left ventricle when the tumor is on the left side and to the septum when it is on the right side resulting in inclusion of these walls in the RT field with consequent ischemic changes after CRT.
It is intriguing that we found arrhythmia to be a significant predictor of cardiac morbidity after CRT. Rice et al. at our institution similarly noted that elderly patients receiving preoperative CRT for esophageal cancer are more likely to develop postoperative atrial arrhythmias than patients who do not receive preoperative CRT (P = 0.004) [22]. Investigators have also found abnormalities in repolarization and an increased frequency of premature ventricular complexes in cancer patients following anterior chest wall irradiation. Orzan et al. [23] found that up to 50% of patients who received anterior chest irradiation can present with some electrocardiogram abnormalities, although the incidence of heart block in these patients is low. Most often, the symptomatic presentations of these abnormalities were complete heart block and syncope. Also, histologic studies suggested that conduction abnormalities are caused by fibrosis in the myocardium adjacent to the conduction system [24, 25]. As fibrosis replaces myocardial tissue, progressive loss of nerve function ensues. Franken et al. investigated the effect of irradiation on myocardial norepinephrine concentration as well as on alpha and beta-adrenergic receptor density in the rat heart [26]. They found a decrease in norepinephrine concentration up to 50%, an increase in alpha-receptor concentration and beta-receptor concentration to a maximum of 210 and 150%, respectively. These studies suggested that RT alters the sympathetic innervation to the heart. The preexistence of arrhythmia prior to CRT probably renders the patient more vulnerable to the deleterious effects of CRT on cardiac innervation. The fact that history of CHF prior to CRT was associated with significant cardiac complications in the total study population was an expected finding.
The contribution of chemotherapy in many of our patient to the cardiac morbidities after RT could not be separately evaluated in our study due to the retrospective nature of the study. However, we feel that our patient population represents the actual clinical population that oncologists manage on a routine daily basis with the majority being treated with CRT and small percentage being treated with RT alone.
One limitation of our study was the small number of patients in the esophageal cancer group. This is due to the limited number of patients who underwent both before and after CRT MPI studies. Further studies with larger numbers of patients are warranted. Another possible limitation in our study is the lack of gating during the rest SPECT part of the MPI since it is performed with Thallium-201 in our dual isotope protocol which does not allow us to evaluate for possible stunned myocardium on the pre-RT versus the post-RT MPI scans.
In conclusion, cardiac morbidity after CRT is more frequently encountered in esophageal cancer than lung cancer patients although the difference did not reach statistical significance. Many of the patients demonstrate new ischemic changes on repeat MPI studies after CRT but only small percentage manifested clinically during a mean flow-up interval of 14.6 and 23.1 month in esophageal and lung cancer group, respectively. Most of the cardiac complications after CRT were related to conduction abnormalities and pericardial effusions. The history of arrhythmia or CHF was found to be significantly associated with future cardiac morbidity after CRT when tested in univairate analysis, but weak predictors in multivariate analysis.
References
Hull MC, Morris CG, Pepine CJ et al (2003) Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 290:2831–2837. doi:10.1001/jama.290.21.2831
Rutqvist LE, Lax I, Fornander T et al (1992) Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 22(5):887–896. doi:10.2172/5375237
Haybittle JL, Brinkley D, Houghton J et al (1989) Postoperative radiotherapy and late mortality: evidence from the cancer research campaign trial for early breast cancer. BMJ 298(6688):1611–1614
Biovin JF, Hutchinson GB, Lubin JH et al (1992) Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer 69(5):1241–1247
Hancock SL, Tucker MA, Hoppe RT (1993) Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 270(16):1949–1955. doi:10.1001/jama.270.16.1949
Patt DA, Goodwin JS, Kuo YF et al (2005) Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol 23(30):7475–7482. doi:10.1200/JCO.2005.13.755
Giordano SH, Kuo YF, Freeman JL et al (2005) Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 97(6):419–424
Darby SC, McGale P, Taylor CW et al (2005) Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300, 000 women in US SEER cancer registries. Lancet Oncol 6(8):557–565. doi:10.1016/S1470-2045(05)70251-5
Harris EER, Correa C, Hwang W-T et al (2006) Late cardiac mortality and morbidity in early-stage beast cancer patients after breast-conservation treatment. J Clin Oncol 24(25):4100–4106. doi:10.1200/JCO.2005.05.1037
Gyenes G, Fornander T, Carlens P et al (1996) Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys 36:899–905. doi:10.1016/S0360-3016(96)00125-3
Hojris I, Sand N, Andersen J et al (2000) Myocardial perfusion imaging in breast cancer patients with or without post-mastectomy radiotherapy. Radiother Oncol 55:163–172. doi:10.1016/S0167-8140(00)00170-5
Hardenbergh PH, Munley MT, Bentel GC et al (2001) Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys 49:1023–1028. doi:10.1016/S0360-3016(00)01531-5
Yu X, Prosnitz R, Zhou S et al (2003) Symptomatic cardiac events following radiation therapy for left-sided breast cancer: possible association with radiation therapy-induced changes in regional perfusion. Clin Breast Cancer 4(3):193–197. doi:10.3816/CBC.2003.n.024
Heidenreich PA, Schnittger I, Strauss HW et al (2007) Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol 25:43–49. doi:10.1200/JCO.2006.07.0805
Carver JR, Shapiro CL, Ng A et al (2007) American Society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25:3991–4008. doi:10.1200/JCO.2007.10.9777
Espey DK, Wu X, Swan J, Wiggins C et al (2007) Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska natives. NCI-SEER report
Palm A, Johansson KA (2007) A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol 46(4):462–473. doi:10.1080/02841860701218626
Bush DA, Slater JD, Shin BB et al (2004) Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 126(4):1198–1203. doi:10.1378/chest.126.4.1198
Wagdi P, Fluri M, Aeschbacher B et al (1996) Cardioprotection in patients undergoing chemo- and/or radiotherapy for neoplastic disease. A pilot study. Jpn Heart J 37(3):353–359
Gayed IW, Liu HH, Yusuf SW et al (2006) The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med 47:1756–1762. doi:10.1007/3-540-30005-8
Gayed IW, Liu HH, Wei X et al (2009) Patterns of cardiac perfusion abnormalities after chemoradiotherapy in patients with lung cancer. J Thorac Oncol 4(2):179–184
Rice DC, Correa AM, Vaporciyan AA et al (2005) Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity. Ann Thorac Surg 79(2):391–397. doi:10.1016/j.athoracsur.2004.08.045
Orzan F, Brusca A, Gaita F et al (1993) Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol 39(2):151–156. doi:10.1016/0167-5273(93)90027-E
Arsenian MA (1991) Cardiovascular sequelae of therapeutic thoracic radiation. Prog Cardiovasc Dis 33(5):299–311. doi:10.1016/0033-0620(91)90022-E
Cohen SI, Bharati S, Glass J et al (1981) Radiotherapy as a cause of complete atrioventricular block in Hodgkin’s disease. An electrophysiological-pathological correlation. Arch Intern Med 141(5):676–679. doi:10.1001/archinte.141.5.676
Franken NA, van der Laarse A, Bosker FJ et al (1992) Time dependent changes in myocardial norepinephrine concentration and adrenergic receptor density following X-irradiation of the rat heart. Int J Radiat Oncol Biol Phys 24(4):721–727. doi:10.2172/5375237
Acknowledgments
The authors would like to thank Robyn Harrell for her contribution to the statistical analysis of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gayed, I., Gohar, S., Liao, Z. et al. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 25, 487–495 (2009). https://doi.org/10.1007/s10554-009-9440-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9440-7