Abstract
Purpose This investigation sought to determine which newly available asynchrony parameter derived from gated myocardial perfusion SPECT (GMPS) systolic wall thickening data best distinguishes patients with left bundle branch block (LBBB) from normal subjects. Methods and materials Emory Cardiac Toolbox (ECTb) algorithms were used to compute left ventricular (LV) global and regional function and perfusion indices with regional contraction phases for 20 patients with LBBB, and in 9 control (CTL) subjects who had no function or perfusion abnormalities. Histogram plots of phase frequencies versus R–R interval times included phase standard deviation (SD), bandwidth (BW), skewness and kurtosis. Z-score asynchrony measures were derived for phases sampled using the conventional 17-segment model. Results In CTLs contraction occurred nearly simultaneously in all segments, while LBBBs exhibited a wide variety of heterogeneous contraction patterns. Global parameters that differed between LBBBs versus CTLs included EF, end-systolic volume and end-diastolic volume, and asynchrony measures that were different included BW, phase SD and z-scores. Z-scores most strongly discriminated LBBBs from CTLs (93% of cases correctly predicted, logistic regression χ2 = 29.7, P < 0.0001). Z-scores, phase SD and lateral–septal wall timing were highly reproducible (r = 0.99, 0.99 and r = 0.87, respectively), with no significant inter-observer differences. Conclusion While traditional global function parameters were different in LBBBs and CTLs, asynchrony parameters characterized LBBB most strongly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congestive heart failure (CHF) is a serious disorder caused by numerous conditions, including coronary disease, cardiomyopathy, hypertension, and valvular heart disease. CHF with left bundle branch block (LBBB) is a serious disorder [1], for which cardiac resynchronization therapy (CRT) with biventricular pacing has been shown to be beneficial [2], particularly if the ejection fraction (EF) is ≤35% and LBBB (QRS duration ≥120 ms) is present [3]. However, as many as 20–30% of patients with CHF and LBBB who meet criteria for implantation do not derive significant clinical benefit from the procedure, and their status may even worsen [4]. New cardiac measurements have been sought to predict clinical responsiveness to CRT that are superior to the purely electrocardiographic and EF criteria now employed [5].
Patients with CHF and LBBB frequently undergo gated myocardial perfusion SPECT (GMPS) imaging to evaluate coronary status and determine LV function and EF [6]. One study has suggested that improvement in visually assessed GMPS lateral to septal wall contraction pattern predicts clinical improvement following CRT [7]. Recently, more advanced GMPS data processing techniques incorporating phase analysis has been used to evaluate left ventricular (LV) synchronicity [8, 9].
In this study we retrospectively analyzed a diverse group of patients with congestive heart failure (CHF) and LBBB due to a variety of etiologies, who are typical of individuals referred for evaluation for possible CRT. The same GMPS data used for their perfusion and function evaluations were also used for systolic wall thickening (SWT) phase analysis to yield measurements of ventricular asynchrony. Our goal was to determine which quantitative parameters generated by SWT most reliably distinguishes patients with CHF and LBBB from normal subjects.
Methods and materials
Patient population
The study group consisted of 20 patients with LBBB who were referred by their physicians for scintigraphic evaluation of myocardial perfusion due to a clinical diagnosis of CHF. The criterion used to identify LBBB was prolonged QRS of at least 120 ms, measured by an experienced cardiologist examining the widest QRS complex recorded on calibrated graph paper. LBBBs had a mean age of 63 ± 14 years; 56% were male. They predominantly had coronary disease (95%), rather than cardiomyopathy (5%). Sixty percent had previous CABG and 55% had prior MI.
There were nine control subjects (CTLs) (age = 73 ± 13 years; 65% male). CTLs consisted of patients referred for evaluation of chest pain, or other symptoms suggestive of CAD, but for whom myocardial perfusion and global and regional ventricular function were normal on both nuclear and echocardiographic studies. CTLs had no ventricular conduction delays on ECG.
All subjects were in sinus rhythm; none had atrial fibrillation. All subjects had technically satisfactory myocardial perfusion studies with adequate synchronization of ECG gating and no evidence of patient motion.
Data acquisition
Scans were acquired after an intravenous injection of 111 MBq (3 mCi) 201Tl, followed by non-gated resting SPECT myocardial perfusion imaging. Exercise or pharmacologic stress was then performed, and 1.11 GBq (30 mCi) of 99mTc-sestamibi was injected at peak stress. Post-stress GMPS imaging was performed 30–60 min later, using one of two commercially available dual-detector gamma cameras to collect GMPS data (“Forte” and “Vertex,” ADAC Laboratories, Milpitas, CA). Data were acquired with patients at rest using low-energy high-resolution (LEHR) collimators in 64 × 64 matrices of pixel size 4.6 mm, at 16 frames per R–R interval, using an R-wave window of ±10% of mean pre-acquisition heart rate. Heart rate data were recorded automatically in image files. Data were acquired for 25 s/projection for 32 projections per detector over a total arc of 180°, from the RAO-45° projection to the LPO-45° projection. Total acquisition time was 15–16 min.
Contraction phase analysis processing
Reconstructed tomographic slices were produced by filtered backprojection, using a pre-processing Butterworth filter (power = 5.0, cutoff = 0.50 cycles/cm). No scatter or attenuation corrections were applied to gated data. Standard Emory Cardiac Toolbox (ECTb) algorithms were used to calculate LV end-diastolic volumes (EDV), end-systolic volumes (ESV) and ejection fractions (EF) for both LBBB and CTL groups [10]. Myocardial perfusion was assessed visually, and ECTb algorithms computed perfusion summed stress scores (SSS), which incorporates information as to the number of standard deviations beyond a normal distribution for an individual patient’s myocardial perfusion distribution [11].
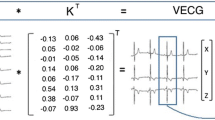
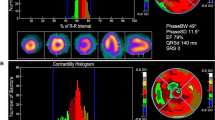
To analyze contraction patterns, modifications were made to ECTb algorithms [8]. The algorithms tested for the possibility of count drop offs near the end of the R–R interval due to minor heart rate variations, and corrected end-frame counts if necessary. Polar perfusion maps were generated for each gated segment of the R–R interval. Counts versus time curves were constructed at each pixel location, and were fit to the principle component of a Fourier series. The peak of the fitted curve corresponded to the time of end-systole, due to the partial volume effect [12]. Systolic phase was expressed as percent of the R–R interval, and equivalently in units of degrees ranging from 0° to 360°. Since phases were computed from the time sequence of polar perfusion maps, they were easily displayed in polar map format (Figs. 1 and 2). Phase histograms were formed by tabulating the number of pixels undergoing peak systolic contraction at each percent of the R–R interval (Figs. 1 and 2). Parameters computed from phase histograms included the time at which the greatest number of pixels were undergoing maximum systolic contraction (peak phase), standard deviation (SD) of contraction phases, phase bandwidth (BW) (defined as the % of the R–R interval for which 95% of contraction phases were observed), skewness and kurtosis.
Phase polar plots
While GMPS phase histogram analyses have been reported previously for LBBBs [9], GMPS spatial distributions of phases of LBBBs have not. To accomplish this, all pixels were assigned to a specific LV segment, corresponding to one of the 17 LV segments in the conventional model used for regional perfusion analysis [13]. The mean phase value for all pixels belonging to a segment was then computed. Polar maps of mean phase for the 17 segments were constructed for each patient (Fig. 3).
(a) Example for a normal subject of regional phase distribution for the LV in a 17-segment model, expressed in units of % of R–R interval, with a maximum 5% difference between phases. (b) LV 17-segment phases for a patient with LBBB, with a 21% difference between earliest versus latest regional phases of contractions
Phase z-scores
To quantify an individual’s degree of LV asynchrony “z-scores” were computed from the pattern among the 17 phases per individual [14]. Z-scores are dimensionless parameters, computed as the difference between phase SD for an individual − mean phase SD for a normal population, and normalized to the standard deviation of phase SD for a normal population. Z-scores >2.0 indicate a probability >95% for the measured parameter falling outside the normal range, indicating that an individual has greater variability among contraction phases of the 17 LV segments than a normal subject.
Lateral–septal wall contraction timing
We computed lateral–septal wall contraction timing differences, previously suggested as an important potential descriptor of asynchrony capable of separating responders from non-responders to CRT [7]. These values were computed as the mean contraction time of the four lateral wall segments, subtracted from mean contraction time of the four septal wall segments. The value was expressed in units of ms, derived using the individual’s heart rate during the acquisition of the gated scintigrams.
Reproducibility
To assess reproducibility of the new GMPS asynchrony measurements we are reporting, a second experienced observer, with no knowledge of clinical information or results obtained by the first observer, applied the algorithms to the original data sets for CTLs and LBBBs.
Data analysis
All values are reported as mean ± 1 standard deviation. Unpaired t-tests were used to test the hypothesis that functional and perfusion parameters were not different between LBBBs and CTLs. Post-hoc power analysis was performed for comparisons between computed parameters for LBBBs versus CTLs. Measurements also were compared among LBBBs with and without MI, CABG, and low LVEF subgroups. ROC analysis was used to determine the threshold of abnormality for each variable to distinguish LBBBs from CTLs, used subsequently by McNemar’s test. Univariate logistic regression determined which single variable most strongly discriminated LBBBs from CTLs. Reproducibility of the new GMPS asynchrony measurements were assessed by linear regression and paired t-tests. A probability (P) value <0.05 was considered to be statistically significant. All statistical tests were 2-tailed.
Results
QRS duration was significantly longer for LBBBs than CTLs (148 ± 17 ms vs. 71 ± 11 ms, P < 0.0001), while heart rates were similar (70 ± 11 bpm vs. 76 ± 15 bpm, P = 0.32). Stress and rest perfusion scans were normal for all CTLs, but perfusion SSS was greater for LBBBs (P = 0.04). Out of twenty patients nine had ischemia. Septal perfusion was lower for stress and rest studies for LBBBs with MI (P = 0.002).
Maximum contraction occurred nearly simultaneously for CTLs, but was dispersed for LBBBs (Fig. 3). Parameters with the greatest differences between LBBBs and CTLs were phase z-score and phase SD (Table 1). Peak contraction phases occurred later in LBBBs with versus without ischemia (144 ± 22° vs. 112 ± 25°, P = 0.007).
There were no differences for measurements between LBBB subgroups with and without MI, and with and without CABG. Other than larger ESVs (P = 0.001), the only difference for the 10 LBBBs with lower EFs (<45%) versus the 10 LBBBs with higher EFs (>45%) was wider BW (168 ± 78° vs. 97 ± 51°, P = 0.03). While the number of subjects was small in these subgroups, post-hoc power analysis indicated <5% chance of incorrectly accepting the null hypothesis regarding significance of differences of quantified variables between these subgroups.
EDV, ESV, histogram BW and histogram peak phase all were significantly less accurate in distinguishing LBBBs from CTLs than phase SDs and z-scores (ROC areas of 76–87% vs. 95–99%, P < 0.01) (Table 1). Z-scores were most strongly associated with LBBB by univariate logistic regression (Table 1). Only one CTL and only one LBBB patient were incorrectly characterized by the z-score (McNemar’s difference = 7%, P = 0.48).
Lateral–septal wall contraction timing differences was similar for LBBBs and CTLs (14 ± 76 ms vs. 3 ± 15 ms, P = 0.86), although the range for LBBBs (−283 to 122 ms) was considerably broader than for CTLs (−12 to 44 ms). Lateral–septal contraction times did not differ between LBBB subgroups with and without MI, CABG or low EF. Thirty-five percent (7/20) of LBBBs exhibited lateral wall contraction delays compared to the septum, as suggested by the literature [7], but 40% (8/20) showed significant septal wall contraction delays, and 25% (5/20) had insignificant lateral–septal wall timing mismatch. CTLs had nearly synchronous septal and lateral wall contractions (Fig. 4). In the three LBBBs with the smallest lateral–septal time differences (−4.0 to 0.0 ms), z-scores were nonetheless significant (2.6–21.4), due to asynchrony among other LV walls. Thus, LBBBs without lateral–septal asynchrony had asynchrony elsewhere, and while infero-septal contraction sometimes preceded antero-basal contraction, there was a very wide range of contraction patterns for LBBBs overall.
To test whether the newer 17-segment asynchrony scores provided independent information, or were just reflective of more traditional measurements, linear regression was performed among variables. Z-score was not related to SSS (r = 0.35, P = 0.14), and was only weakly related to EF (r = 0.57, P = 0.01). Subjects with z-score >2 were not the same as those with SSS > 4 (McNemar’s difference = 28%, P = 0.03), nor were they the same as those with EF < 50% (21% difference, P = 0.04). QRS duration for LBBBs was uncorrelated with histogram or asynchrony measures, and correlated only weakly with EDV (r = 0.59, P = 0.01).
Inter-observer reproducibility was r = 0.99, 0.99 and 0.87 for z-scores, phase SD and lateral–septal wall timing, respectively (P < 0.0001). There were no differences between measurements of the two observers for any of these parameters (t-test P = NS), consistent with previously reported reproducibility for phase histogram measurements [15].
Discussion
The principle finding of this investigation is that z-scores and 17-segment phase SDs, asynchrony measures that incorporate information from all left ventricular segments, were the most effective parameters in detecting global asynchrony. Because of the wide variety of asynchrony patterns among patients with CHF and LBBB, measures focusing on contraction delays in one direction, such as lateral–septal timing differences, may be less sensitive. The z-score, because it takes into account phase data from all 17 LV segments, is more likely to be reflective of the total degree of dyssynchrony of ventricular contraction. In that sense, it is analogous to a summed stress score, used to reflect the total burden of jeopardized myocardium in quantification of SPECT perfusion imaging [11]. Moreover, the z-score can be referenced to an age-matched population found to be free of heart disease, and thus provides a direct measure of asynchrony beyond the variations seen in normal patients. This parameter has proven useful in quantifying asynchrony from segmental gated blood pool SPECT phase measurements [14, 16], but has not been previously employed in the analysis of GMPS data. These are genuinely new physiological measurements distinct from conventional LV perfusion and function parameters, providing additional information as to cardiac performance not previously available from analysis of GMPS data.
We found that the sequence of contractions was less coordinated in LBBBs than in CTLS. In CTLs, cardiac contraction was predominantly synchronous, with <5% difference in time to peak systolic phase for all left ventricular wall segments. In LBBBs, contraction was significantly less coordinated. Moreover, the specific patterns of LV contraction and asynchrony in LBBBs varied widely, with no standard pattern discernable. Descriptors that incorporated information from all LV segments, the phase z-scores and phase SD, were best able to identify LBBBs with asynchrony, and provide the best separation between that group and CTLs.
Volume and EF values for our CTLs were consistent with values previously reported using ECTb algorithms for subjects with low likelihood of CAD [17], and our phase histogram parameters for CTLs were consistent with values published for the same ECTb algorithms for a group of 157 normal subjects [9]. For CTLs, z-scores were close to 0.0 ± 1.0, as expected [14], and phase SD was consistent with values found in gated blood pool SPECT investigations for normal subjects [16]. Most histogram values in LBBBs in the current investigation were significantly greater, i.e. more abnormal, than those reported using the same ECTb algorithms in an earlier study of 33 patients [9]. This probably reflects the wide variety of mechanisms that produce CHF and LBBB and the heterogeneity of the patient populations [1].
Initial studies of CRT indicated that reducing ventricular asynchrony by bi-ventricular pacing improved long term prognosis, and that intra-ventricular conduction delays and LBBB were strong predictors of clinical response [2]. However, using purely ECG criteria to determine eligibility for treatment results in a 20–30% clinical non-response rate [4], and patients with narrow QRS and abnormal contraction may also respond to CRT [18]. This led to efforts to more adequately define ventricular asynchrony noninvasively. Echocardiography with tissue Doppler imaging has been used to visualize the rate and timing of wall motion in different LV segments. Yu et al. studied 54 patients undergoing CRT and measured time to peak systole in 12 LV segments [19]. In that investigation, the parameter most closely predicting a clinical response to CRT was the standard deviation of time to peak myocardial contraction, a descriptor analogous to phase SDs and z-scores in our study. These Doppler echo data thus support the concept that to adequately quantify LV asynchrony, a parameter must express the global variability of the LV contraction pattern, rather than focus on conduction delay in any single direction across the ventricle.
Study limitations
It was assumed that systolic wall thickening calculations were reflective of resting function, and were unaffected by ischemia, even though SWT phase measurements were performed on post-stress gated images. We cannot exclude the possibility that there could have been some transient residual wall thickening abnormalities in some patients with ischemia [20]. If so, it is possible that this could magnify differences in computed parameters between CTLs and patients with myocardial ischemia.
Also, even though power analysis reported in Table 1 indicated sufficient power, our report must be considered as a preliminary investigation as the sample sizes are small. It is possible that a larger sample size would have revealed a clearer separation between patient subgroups.
Tailoring CRT to the specific asynchrony pattern of an individual may improve clinical efficacy of the procedure [21]. It has not yet been demonstrated that GMPS SWT measurements actually will provide information helpful to CRT planning in patients with LBBB. GMPS BW has been found to predict response to CRT in patients with heart failure [22, 23]. Studies of GMPS parameters specific to recovery of patients with LBBB, which has many etiologies [1], have not yet been conducted. Only the analysis of SWT parameters pre-CRT versus post-CRT in clinical outcomes studies in patients with LBBB, specifically, will be capable of resolving such issues in the future.
Conclusion
Systolic wall thickening phase analysis of gated myocardial perfusion SPECT data can identify contraction phase abnormalities in patients with conduction delays, and provides parameters such as phase z-scores that are likely to prove useful for quantifying LV asynchrony. Among patients with LBBB, we found no single aberrant pattern of phases, but rather a wide variety of abnormal phase patterns.
Abbreviations
- BW:
-
Bandwidth
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery disease
- CTL:
-
Control subject
- CHF:
-
Congestive heart failure
- CRT:
-
Cardiac resynchronization therapy
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- EF:
-
Ejection fraction
- GMPS:
-
Gated myocardial perfusion SPECT
- LBBB:
-
Left bundle branch block
- LV:
-
Left ventricular
- MI:
-
Myocardial infarction
- ms:
-
Millisecond
- SSS:
-
Summed stress score
- SWT:
-
Systolic wall thickening
References
Jeong JH, Kim JH, Park YH et al (2004) Incidence of and risk factors for bundle branch block in adults older than 40 years. Korean J Intern Med 19:171–178
Cleland JG, Daubert JC, Erdmann E et al (2005) Cardiac Resynchronization-Heart Failure (CARE-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352:1539–1549. doi:10.1056/NEJMoa050496
Gregoratos G, Abrams J, Epstein AE et al (2002) ACC/AHA/NASPE (2002) guideline update for implantation of cardiac pacemakers and antiarrhythmia devices a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee on Pacemaker Implantation). Circulation 106:2145–2161. doi:10.1161/01.CIR.0000035996.46455.09
Leclercq C, Kass DA (2002) Retiming the failing heart: principles and current clinical status of cardiac resynchronization. J Am Coll Cardiol 39:194–201. doi:10.1016/S0735-1097(01)01747-8
Yu CM, Chau E, Sanderson JE et al (2002) Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 105:438–445. doi:10.1161/hc0402.102623
Candell-Riera J, Oller-Martínez G, Pereztol-Valdes O et al (2003) Usefulness of myocardial perfusion SPECT in patients with left bundle branch block and previous myocardial infarction. Heart 89:1039–1042. doi:10.1136/heart.89.9.1039
Tsurugaya H, Tada H, Toyama T et al (2004) Usefulness of quantitative gated single-photon emission computed tomography to evaluate ventricular synchrony in patients receiving biventricular pacing. Am J Cardiol 94:127–130. doi:10.1016/j.amjcard.2004.03.044
Chen J, Garcia EV, Folks RD et al (2005) Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dysynchrony. J Nucl Cardiol 12:687–695. doi:10.1016/j.nuclcard.2005.06.088
Trimble MA, Borges-Neto S, Smallheiser S et al (2007) Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol 14(3):298–307. doi:10.1016/j.nuclcard.2007.01.041
Faber TL, Cooke CD, Folks RD et al (1999) Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med 40:650–659
Hachamovitch R, Berman DS, Kiat H et al (1996) Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation 93:905–914
Galt JR, Garcia EV, Robbins WL (1990) Effects of myocardial wall thickness on SPECT quantification. IEEE Trans Med Imaging 9:144–150. doi:10.1109/42.56338
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 18:539–542
Vilain D, Daou D, Casset-Senon D, Faraggi M, Le Guludec D (2001) Optimal 3-dimensional method for right and left ventricular Fourier phase analysis in electrocardiography-gated blood-pool SPECT. J Nucl Cardiol 8:371–378. doi:10.1067/mnc.2001.114151
Trimble MA, Velazquez EJ, Adams GL et al (2008) Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun 29(4):374–381
Nichols KJ, Van Tosh A, De Bondt P et al (2008) Normal limits of gated blood pool SPECT count-based regional cardiac function parameters. Int J Cardiovasc Imaging. http://www.springerlink.com/content/1569–5794
Nichols K, Santana CA, Folks R et al (2002) Comparison between “ECTb” and “QGS” for assessment of left ventricular function from gated myocardial perfusion SPECT. J Nucl Cardiol 9:285–293. doi:10.1067/mnc.2002.121449
Yu CM, Chan YS, Zhang Q et al (2006) Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol 48(11):2251–2257. doi:10.1016/j.jacc.2006.07.054
Yu CM, Fung JW, Zhang Q et al (2004) Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 110(1):66–73. doi:10.1161/01.CIR.0000133276.45198.A5
Johnson LL, Verdesca SA, Aude WY et al (1997) Postischemic stunning can affect left ventricular ejection fraction and regional wall motion on post-stress gated sestamibi tomograms. J Am Coll Cardiol 30(7):1641–1648. doi:10.1016/S0735-1097(97)00388-4
Alonso C, Leclercq C, Victor F et al (1999) Electrocardiographic predictive factors of long-term clinical improvement with multisite biventricular pacing in advanced heart failure. Am J Cardiol 84:1417–1421. doi:10.1016/S0002-9149(99)00588-3
Henneman MM, Chen J, Dibbets-Schneider P et al (2007) Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med 48:1104–1111. doi:10.2967/jnumed.107.039925
Chen J, Henneman MM, Trimble MA et al (2008) Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol 15:127–136. doi:10.1016/j.nuclcard.2007.11.004
Acknowledgements
We would like to thank Jing Han for her expert assistance with the statistical analysis reported for this project. This work was supported by a grant from the Saint Francis Research Foundation. Some of the authors (Chen, Garcia) receive royalties from the sale of Phase Analysis tool with the Emory Cardiac Toolbox.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nichols, K.J., Van Tosh, A., Siddiqi, S. et al. Gated myocardial perfusion SPECT asynchrony measurements in patients with left bundle branch block. Int J Cardiovasc Imaging 25, 43–51 (2009). https://doi.org/10.1007/s10554-008-9354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-008-9354-9