Abstract
Objective To prospectively compare cardiac ventricular measurements from non-gated CT and end-diastolic ECG-gated CT in patients with acute pulmonary embolism (PE). Materials and methods With institutional review board approval, 30 adult patients (16 female, mean age = 56 years, range = 26–77 years) underwent ECG-gated cardiac CT within 36 h of their CT diagnosis of acute PE to assess the right ventricle (RV). The axial and reformatted four-chamber ventricular diameters, areas and volumes were measured for both the non-gated CT and the ECG-gated CT in end-diastole and end-systole. Spearman’s rank correlation coefficient (RCC) was calculated to compare measurements from the non-gated CT to the gated end-diastolic measurements. The median absolute differences between the gated and non-gated measurements relative to the gated measurements were provided to summarize the degree to which the two measurements differ. A statistical model was constructed to test for potential improvement in specificity for the prediction of 30-day mortality after acute PE using right ventricular measurements from ECG-gated CT versus non-gated CT. Results The RCC (0.90 confidence interval) for non-gated and ECG-gated end-diastolic four-chamber and axial RV/LV diameter ratios were 0.83 (0.68–0.90) and 0.88 (0.74–0.95). The median absolute percent differences suggested a high degree of concordance between gated and non-gated measurements. The statistical model predicted that measuring the RV/LV diameter ratio from end-diastole using ECG-gated CT rather than non-gated CT would yield a potential improvement in specificity for death after PE of 0.035 (0.020–0.060) for axial diameter ratios and 0.035 (0.020–0.055) for four-chamber diameter ratios. Conclusion The benefit from a separate ECG-gated CT scan for the evaluation of RV ventricular diameter, area, and volume measurements is minimal and does not justify its routine clinical use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Risk stratification after acute pulmonary embolism (PE) is important because it allows for appropriate clinical management [1]. As PE elevates pulmonary vascular resistance, right ventricular afterload increases. Left unchecked, this may lead to a dilated, hypokinetic right ventricle that ultimately causes systemic hypotension and cardiac arrest [2]. Echocardiography is the established imaging modality for risk stratification after PE; several studies suggest that echocardiographic right ventricular dilatation or right ventricular wall hypokinesis may identify those at high risk for right ventricular failure and death [3–6].
Computed tomography (CT) is the first line diagnostic study for PE [7, 8]. As the entire heart is imaged on nearly every PE protocol CT, there has been substantial interest in using the diagnostic CT pulmonary angiogram (CTPA) to assess the right ventricle and by extension to assess prognosis after PE [9]. Several studies support an increased RV/LV diameter ratio, as measured in the standard axial or reformatted four-chamber views, as a predictor of short term mortality after PE [10–22], though the use of CTPA for this purpose remains controversial [23]. For patients with a negative prior examination, measuring the interval increase in the RV/LV ratio improves specificity since the patient may have preexisting RV enlargement before PE [24].
In routine imaging for patients with suspected PE, CT data is acquired without ECG gating. One reason is that pulmonary arterial pulsation artifact is usually small [25]. For visualization of the pulmonary arteries, breathing artifacts are typically more significant than pulsation artifacts [26], and ECG-gated scans generally have a longer acquisition time than comparable protocols without ECG-gating. The notable exception is single gantry rotation ECG-gated wide area detector axial CT [27]. The second and perhaps more significant reason to avoid ECG gating in CTPA is the greater patient radiation dose. Thus, from the perspective of detecting a filling defect in the pulmonary arterial system, the support for implementing ECG-gating into routine protocols is relatively weak. Moreover, CTPA is widely used because of the clinical and laboratory challenges in excluding patients with clinically suspected PE. Hence, the large majority of studies are negative for PE, and the cumulative increased radiation to those patients who do not have PE can be substantial.
When considering CTPA as an exam that not only makes the diagnosis of PE but also gives prognostic information via the RV/LV measurements, there are potential advantages of incorporating ECG-gating, despite the increased radiation. The first potential advantage is that ECG-gating minimizes and in most cases eliminates cardiac motion artifact. Thus, measurements of the ventricular diameters can be, in theory, more precise. The second advantage is that retrospective ECG-gating can be performed with CT acquisition throughout the cardiac cycle. This information enables RV cine evaluation so that wall motion and RV function can be assessed, akin to echocardiography. The purpose of this study is to prospectively compare cardiac ventricular measurements from non-gated CT and end-diastolic ECG-gated CT in patients with acute pulmonary embolism (PE).
To achieve significant results with a modest prospective patient cohort we have created a statistical model that reflects non-gated cardiac CT versus ECG-gated CTPA. Specifically, we assume that the ECG-gated exam has less motion artifact and model the motion introduced by the absence of gating as an error in measurement to test the hypothesis that ECG-gated exams have improved specificity for predicting 30-day mortality after PE.
Materials and methods
Study subjects
The institutional human research committee approved prospective ECG-gated CT dedicated to evaluate the right ventricle. Thirty adult patients (16 female, mean age = 56 years, range = 26–77 years) diagnosed with PE by routine non-gated CT were enrolled between July 2005 and February 2006 at a single institution. Each subsequently received a second, ECG-gated CT to evaluate their right ventricle within 36 h of the first non-gated CT examination.
Image acquisition
Non-gated CTPA was performed according to our standard institutional protocol. Four, sixteen and sixty-four slice multidetector (MD) CT scanners (Siemens Medical Systems, Erlangen, Germany) were used with 120 kV and an effective mAs ~200. One-hundred and twenty-five cc of iodinated contrast (Ultravist 370, Berlex, Richmond, CA) were administered via a power injector. Bolus tracking was employed on the main PA, with image acquisition at 1.0–1.25 × 0.75–1.0 mm.
The ECG-gated CT scans were performed on 16 (gantry rotation time 420 ms) and 64 (gantry rotation time 330–370 ms) slice MDCT scanners (Siemens) at 120 kV and an effective mAs of ~300–400. Fifty cc of iodinated contrast (Ultravist 370) were administered via a power injector. Bolus tracking was employed, with image acquisition at 1.5 × 1.0 mm. The 1 mm axial images were reconstructed at every 12.5% of the RR interval, yielding eight phases. The two phases corresponding to end-diastole and end-systole were visually identified and used for image post-processing and analysis.
Image post-processing and analysis
Each patient had three series of images: the non-gated diagnostic CTPA, the ECG-gated end-diastolic images, and the ECG-gated end-systolic images. For all three series, four-chamber reformations designed to standardize the RV/LV diameter ratios [28] were rendered on a dedicated 3D workstation (Leonardo Siemens, Erlangen, Germany). The left and right ventricular diameters, areas, and volumes were measured. Ventricular diameters were measured as the greatest distance between the interventricular septum and the free ventricular wall along the short axis of the heart. For the four-chamber measurements, the left and right ventricular diameters were measured on a single image best depicting the four-chamber view [20]. For the axial view, the diameter of each ventricle was measured on the axial level where it was largest [19]. Ventricular areas were measured on the same images as their respective diameters and were defined by the border of the valve plane, endocardium and interventricular septum, inclusive of papillary muscles. For the measurement of volumes, a series of consecutive 3 mm short axis images was reformatted. Endocardial contours inclusive of papillary muscle and trabeculation were manually traced then automatically summed by the workstation to calculate ventricular cavity volumes [29–32]. For the ECG-gated scans, ventricular ejection fractions were calculated from end-systolic and end-diastolic volume measurements.
Statistical analysis
For the RV/LV diameter, area and volume ratios, Spearman’s Rank Correlation Coefficients (RCC) with 0.90 confidence intervals were calculated for non-gated versus gated end-diastolic images. In addition, the median absolute differences between the gated and non-gated measurements relative to the gated measurements were provided to summarize the degree to which the two measurements differ. A statistical model estimated the potential increase in specificity of gated end-diastolic measurements compared to non-gated measurements of diameter, area and volume ratios. The model made three assumptions. The first assumption was that the log-transformed non-gated measurements were the log-transformed gated measurements plus an independent normally distributed random error, the latter attributed to motion inherent in measurements obtained from non-gated images compared to similar measurements obtained from a gated exam. The second assumption was that the RV/LV diameter ratios of patients with and without RV dysfunction follow log-normal distributions with different means but the same variance. The third assumption, extracted from the literature [20], was that that four-chamber RV/LV diameter ratios achieve a sensitivity = 0.78 and specificity = 0.38 to predict 30-day mortality (prevalence = 0.15) for a four-chamber RV/LV diameter ratio >0.9. To our knowledge, there is no CT data that associate area and volume ratios with death after PE. Thus, the area and volume ratios used the same assumptions for sensitivity and specificity. These assumed models lead to binormal ROC curves [33, 34] for both gated and non-gated measurements. We use the estimated binormal ROC curves to calculate the potential improvement in specificity attributed to using the gated end-diastolic measurements. The same analysis was performed for ventricular area and volume ratios. The bootstrap methods were used to obtain confidence intervals for all the parameters of interest [35].
Results
The rank correlation coefficients (Table 1) demonstrate a high level of correlation between non-gated versus gated end-diastolic measurements. The RCC (0.90 CI) of the axial RV/LV diameter ratio was 0.88 (0.74–0.95). The RCC of the four-chamber RV/LV diameter ratio was 0.83 (0.68–0.90). Furthermore, the median absolute percent differences (Table 2) also suggest a high degree of concordance between the gated and non-gated measurements. On average, the two measurements differ by 8.0% (0.90 CI: [5.4%, 10.6%]) for the axial RV/LV diameter ratio and by 6.8% (0.90 CI: [2.1%, 11.6%]) for the four-chamber RV/LV diameter ratio. These findings suggests limited or no potential benefit from ECG gating for the assessment of ventricular diameter ratios.
Assuming that non-gated diameter ratios achieve a specificity of 0.38 at a sensitivity of 0.78, the predicted specificity of end-diastolic ECG-gated diameter ratios ranged from 0.42 to 0.46 (Table 3). The predicted gain in specificity ranged from 0.035 to 0.083, indicating that diameter, area, and volume measurements obtained from ECG-gated CT provides limited potential improvement over non-gated CT for predicting 30-day mortality.
The mean LV ejection fraction was 0.60 ± 0.068 (range 0.42–0.75), and the mean RV ejection fraction was 0.48 ± 0.10 (range 0.33–0.65).
Discussion
The cost of evaluating the right ventricle with a separate ECG-gated CT scan includes the administration of iodinated contrast, radiation exposure, and time. The potential benefit is ventricular measurements without motion artifact. We demonstrate only minimal incremental change from measurements of ventricular diameter, area, and volume from ECG-gated acquisition. To date, the most widely studied measurements are the axial and four-chamber diameter ratios. The high correlation between non-gated and gated end-diastolic diameter ratios suggests minimal benefit from performing ECG gating for these measurements. This is further supported by the minimal improvement in specificity for 30-day mortality using the statistical model. Our study demonstrates correlation between non-gated and gated end-diastolic area and volume measurements. The correlation was not as strong as that for the diameter ratios, probably reflecting more variability in these measurements (Figs. 1, 2).
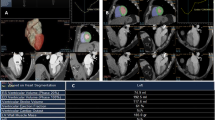
Four-chamber views shown with the ventricular diameter measurements obtained in ECG-gated end-systole (a) (top) and end-diastole (b) (middle) in a 59-year-old man diagnosed with acute PE. (c) (bottom) four-chamber ventricular diameter measurements obtained from the non-gated CT pulmonary angiogram in the same patient
Four-chamber views shown with the ventricular diameter measurements obtained in ECG-gated end-systole (a) (top) and end-diastole (b) (middle) in a 57-year-old woman diagnosed with acute PE. (c) (bottom) four-chamber ventricular diameter measurements obtained from the non-gated CT pulmonary angiogram in the same patient
In a related study by Dogan et al. [36], 66 patients, 29 of whom had PE, were examined with non-gated and ECG-gated CT. The mean four-chamber RV/LV diameter ratios and RV end-systolic volumes were higher in patients with PE than those with no PE. Furthermore, end-systolic RV volumes and end-systolic and end-diastolic RV/LV volume ratios were significantly different between those patients with central versus peripheral PE. These important findings suggest that diastolic and systolic volumes help assess the severity of PE. The methods of our study differ in that we directly compared non-gated and ECG-gated RV/LV diameter ratios as predictors of mortality after PE. To date, the RV/LV diameter ratio is the only ventricular CT measurement directly associated with mortality after PE [9].
The statistical model achieved narrow confidence intervals to compare measurements with and without ECG-gating using a relatively small patient cohort. However, a limitation of this study is that this sample is too small to study the potential utility of ventricular ejection fraction and wall motion abnormalities. To explore the full potential of ECG-gated exams, morphologic parameters could be used in conjunction with right ventricular function in a large enough sample where there are a significant number of patients with 30-day mortality. In addition, dual source CT scanners achieve an 83 millisecond temporal resolution so that the cine assessment of the ventricular walls to assess the RV can more closely approximate echocardiography. The potential drawback of this method is that retrospective ECG-gating is required, increasing the radiation dose in comparison to prospective ECG gated methods.
In conclusion, ventricular measurements obtained from non-gated CT images correlate with their ECG-gated end-diastolic counterparts. The correlation is strongest with RV/LV diameter ratios. For ventricular diameters, areas, and volumes, modeling the cardiac motion inherent in non-gated CTPA as an error with respect to measurements made from ECG-gated studies shows minimal improvement in specificity for predicting 30-day mortality after PE. Larger patient samples focused on PE related mortality can more fully address the impact of incorporating ECG-gated CT into the evaluation of RV dysfunction and the tradeoffs associated with the associated radiation exposure.
References
Konstantinides S (2005) Pulmonary embolism: impact of right ventricular dysfunction. Curr Opin Cardiol 20:496–501
Goldhaber SZ, Elliott CG (2003) Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation 108:2726–2729
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101:2817–2822
Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2005) Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 165:1777–1781
Goldhaber SZ (2002) Echocardiography in the management of pulmonary embolism. Ann Intern Med 136:691–700
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L (1997) Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 134:479–487
Quiroz R, Kucher N, Zou KH, Kipfmueller F, Costello P, Goldhaber SZ, Schoepf UJ (2005) Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA 293:2012–2017
Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Leeper KV Jr, Popovich J Jr, Quinn DA, Sos TA, Sostman HD, Tapson VF, Wakefield TW, Weg JG, Woodard PK (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354:2317–2327
Ghaye B, Ghuysen A, Bruyere PJ, D’Orio V, Dondelinger RF (2006) Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics 26:23–39 (discussion 39–40)
Reid JH, Murchison JT (1998) Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clin Radiol 53:694–698
Collomb D, Paramelle PJ, Calaque O, Bosson JL, Vanzetto G, Barnoud D, Pison C, Coulomb M, Ferretti G (2003) Severity assessment of acute pulmonary embolism: evaluation using helical CT. Eur Radiol 13:1508–1514
Contractor S, Maldjian PD, Sharma VK, Gor DM (2002) Role of helical CT in detecting right ventricular dysfunction secondary to acute pulmonary embolism. J Comput Assist Tomogr 26:587–591
Lim KE, Chan CY, Chu PH, Hsu YY, Hsu WC (2005) Right ventricular dysfunction secondary to acute massive pulmonary embolism detected by helical computed tomography pulmonary angiography. Clin Imaging 29:16–21
Quiroz R, Kucher N, Schoepf UJ, Kipfmueller F, Solomon SD, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation 109:2401–2404
Ghuysen A, Ghaye B, Willems V, Lambermont B, Gerard P, Dondelinger RF, D’Orio V (2005) Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax 60:956–961
He H, Stein MW, Zalta B, Haramati LB (2006) Computed tomography evaluation of right heart dysfunction in patients with acute pulmonary embolism. J Comput Assist Tomogr 30:262–266
Mansencal N, Joseph T, Vieillard-Baron A, Langlois S, El Hajjam M, Qanadli SD, Lacombe P, Jardin F, Dubourg O (2005) Diagnosis of right ventricular dysfunction in acute pulmonary embolism using helical computed tomography. Am J Cardiol 95:1260–1263
Araoz PA, Gotway MB, Trowbridge RL, Bailey RA, Auerbach AD, Reddy GP, Dawn SK, Webb WR, Higgins CB (2003) Helical CT pulmonary angiography predictors of in-hospital morbidity and mortality in patients with acute pulmonary embolism. J Thorac Imaging 18:207–216
van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, de Roos A, Huisman MV (2005) Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology 235:798–803
Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation 110:3276–3280
Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, Gevenols P, Dondelinger RF (2006) Severe pulmonary embolism: pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology 239:884–891
Wittram C (2007) How I do it: CT pulmonary angiography. AJR Am J Roentgenol 188:1255–1261
Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN (2007) Pulmonary embolism: prognostic CT findings. Radiology 242:889–897
Lu MT, Cai T, Ersoy H, Whitmore AG, Quiroz R, Goldhaber SZ, Rybicki FJ (2008) Interval increase in right-left ventricular diameter ratios at CT as a predictor of 30-day mortality after acute pulmonary embolism: initial experience. Radiology 246:281–287
Marten K, Engelke C, Funke M, Obenauer S, Baum F, Grabbe E (2003) ECG-gated multislice spiral CT for diagnosis of acute pulmonary embolism. Clin Radiol 58:862–868
Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC (2004) CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics 24:1219–1238
Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, Ersoy H, Mather RT, Judy PF, Cai T, Coyner K, Schultz K, Whitmore AG, Di Carli MF (2008) Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging 24:535–546
Lu MT, Ersoy H, Whitmore AG, Lipton MJ, Rybicki FJ (2007) Reformatted four-chamber and short-axis views of the heart using thin section (≤2 mm) MDCT images. Acad Radiol 14:1108–1112
Delhaye D, Remy-Jardin M, Teisseire A, Hossein-Foucher C, Leroy S, Duhamel A, Remy J (2006) MDCT of right ventricular function: comparison of right ventricular ejection fraction estimation and equilibrium radionuclide ventriculography, part 1. AJR Am J Roentgenol 187:1597–1604
Remy-Jardin M, Delhaye D, Teisseire A, Hossein-Foucher C, Duhamel A, Remy J (2006) MDCT of right ventricular function: impact of methodologic approach in estimation of right ventricular ejection fraction, part 2. AJR Am J Roentgenol 187:1605–1609
Dogan H, Kroft LJ, Bax JJ, Schuijf JD, van der Geest RJ, Doornbos J, de Roos A (2006) MDCT assessment of right ventricular systolic function. AJR Am J Roentgenol 186:S366–S370
Lembcke A, Dohmen PM, Dewey M, Klessen C, Elgeti T, Hermann KG, Konertz WF, Hamm B, Kivelitz DE (2005) Multislice computed tomography for preoperative evaluation of right ventricular volumes and function: comparison with magnetic resonance imaging. Ann Thorac Surg 79:1344–1351
Swets JA (1986) Indices of discrimination or diagnostic accuracy: their ROCs and implied models. Psychol Bull 99:100–117
Hanley JA (1996) The use of the ‘binormal’ model for parametric ROC analysis of quantitative diagnostic tests. Stat Med 15:1575–1585
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall, New York
Dogan H, Kroft LJ, Huisman MV, van der Geest RJ, de Roos A (2007) Right ventricular function in patients with acute pulmonary embolism: analysis with electrocardiography-synchronized multi-detector row CT. Radiology 242:78–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, M.T., Cai, T., Ersoy, H. et al. Comparison of ECG-gated versus non-gated CT ventricular measurements in thirty patients with acute pulmonary embolism. Int J Cardiovasc Imaging 25, 101–107 (2009). https://doi.org/10.1007/s10554-008-9342-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-008-9342-0