We have conducted experiments on noncatalytic and catalytic aquathermolysis of high-viscosity heavy crude oil from the Ashal’cha field (Tatarstan) in the presence of a crude oil-soluble nickel- and cobalt-containing catalyst, a proton donor, and a rock-forming mineral. We have identified the characteristic features of the change in the constituent composition, the hydrocarbon composition, and the fractional composition, the rheological properties of the crude oils, the average molecular weight of the asphaltenes for catalytic and noncatalytic conversion processes. For catalytic aquathermolysis, we established significant de novo formation of light 70°C-250°C fractions (by 23 wt.%), n-alkylbenzenes, an increase in the oil content by a factor of 1.3, a decrease in the resin content by a factor of 1.7, and a decrease in the viscosity by 98 rel.%. The major difference between the conversion of crude oil in the presence of the catalyst and the proton donor involves activation of degradation reactions at C–C, C–N, C–O, C–S bonds and blocking of polymerization reactions and accordingly less coke formation. We observed sorption of the catalyst components on rock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Utilization of reserves of high-viscosity heavy oils and natural bitumens, which are an important resource for stabilization of oil production, is complicated by the impossibility of refining the indicated crude in current oil refineries, which were designed for refining conventional crude oils. Accordingly, there is interest in scientific research aimed at upgrading the heavy oils and natural bitumens to prepare them for refining. Methods for preparing heavy oils and natural bitumens in the fields can include in particular in situ upgrading during recovery by steam stimulation (aquathermolysis) [1, 2], and also aqueous pyrolysis of natural bitumens in subcritical and supercritical water in a reactor [3, 4].
Conversion of crude oil during steam stimulation has been observed in comparative studies of Ashal’cha crude oil samples recovered by natural flow and by using steam stimulation [5, 6]. Crude oil recovered by steam stimulation is characterized by a higher content of light fractions, lower density, cokability, and content of sulfur, VO-porphyrins, and resins. This has stimulated interest in laboratory simulation of hydrothermal conversions of heavy oils [7–14]. It has been shown that hydrothermal treatment leads to degradation of the least stable C–O, C–S, C–N bonds, detaching peripheral fragments from the resin/asphaltene molecules with de novo formation of oils/resins [6–11].
During aquathermolysis, water acts not only as a heat transfer agent but also as a solvent for organic compounds [15], which is due to the decrease in the dielectric constant as the temperature is raised, and also acts as a catalyst and reagent owing to the decrease in the logarithm of the ion product and accordingly a simultaneous increase in the acid and base properties [16]. In this connection, according to [17–19], the major reactions for conversion of heteroatomic compounds in water at high temperature are thermal cracking and hydrolysis. As shown by the calculations, hydrolysis reactions of C–S, C–O bonds in aliphatic sulfides and esters are thermodynamically possible in the temperature range for steam stimulation [20].
For intensification of conversions of heavy oils and natural bitumens during their in situ upgrading based on the aquathermolysis process, it has been proposed that we use proton donors [21] and different types of catalysts for the hydrogenolysis and hydrogenation reactions: metal nanoparticles [22], water-soluble [23] and crude oil-soluble [24, 25] transition metal compounds. We especially should point out [23, 26–28], describing the results of field tests of aquathermolysis in which a decrease in the viscosity of the recovered oil was detected as well as a decrease in its content of sulfur, resin/asphaltene compounds, and an increase in oil recovery. This suggests that a promising approach is to look for possibilities of intensification of the aquathermolysis process and to study the mechanism for conversions of the components in heavy oils and natural bitumens in catalytic and noncatalytic in situ conversions.
The aim of this work is to study the nature of conversions of heavy oils in catalytic and noncatalytic aquathermolysis as applied to in situ and “surface” upgrading. The object of investigation was crude oil from the Ashal’cha field (Tatarstan). Kaolin clay played the role of the rock-forming mineral. We chose to conduct the experiments in the presence of rock first of all because of the catalytic effect of minerals in aquathermolysis processes [29–31] and secondly because of the possible sorption properties relative to the high molecular weight components of the crude oil and the metals [30].
As the crude oil-soluble catalyst, we used nickel and cobalt carboxylates, which are readily soluble in crude oil, gasoline, and tetralin. The catalyst was added to the crude oil in a solution of the proton donor (tetralin) in a total amount of 0.3 wt.% of the metals based on the crude oil. The catalytic aquathermolysis experiments were run in a 1 L autoclave at a temperature of 300°C for 5 hours, with initial air pressure 1 atm. A mixture of the crude oil, kaolin clay, tetralin, and water in 100:100:5:43 ratio (weight of crude oil, 100 g) was loaded into the autoclave. In parallel, we ran an experiment without the catalyst and tetralin (noncatalytic aquathermolysis), all other conditions unchanged.
The kaolin clay removed from the reactor was subjected to extraction in a Soxhlet apparatus with benzene, in order to separate conversion products from the kaolin clay and the water. The asphaltenes were separated by precipitation with a 40-fold excess of petroleum ether (boiling point 30°C-70°C). Maltenes were separated by liquid adsorption chromatography on ASK silica gel; the oils and the resins (benzenesoluble and alcohol/benzene-soluble) were eluted respectively by a mixture of petroleum ether and CCl4, by benzene, and by a mixture of benzene + isopropyl alcohol.
The IR spectra of extracts from the rocks were recorded on a Vector-22 (Bruker) Fourier-transform IR spectrophotometer in the range 4000-400 cm–1 with resolution 4 cm–1. The elemental composition of the crude oil and asphaltenes was determined by ashing weighed samples on a CHN-3 analyzer at a temperature of 1000°C. The paramagnetic properties of the asphaltenes were studied on an SE/X-2544 EPR spectrometer (RadioPAN, Poland). The microelemental composition of the rock, crude oil, and asphaltenes was analyzed by x-ray fluorescence on an EDX-800HS2 spectrometer. The rock was analyzed by differential thermal analysis on a Q-1500D thermal analyzer (MOM) in the temperature interval 200°C-1000°C with furnace heating rate 10 degrees/minute. The atmosphere in the furnace was stationary air. To determine the molecular weight of the asphaltenes, we used matrix-assisted laser desorption/ionization (MALDI). The studies were conducted on a BRUKER model Ultraflex III TOF/TOF mass spectrometer with time-of-flight analyzer in a matrix of 2,5-dihydroxybenzoic acid. The rheological studies of the crude oil were done on a Rheotest RN 4.1 automatic rotary viscometer at a temperature of 5°C in the shear rate interval 1–370 s-1. The stability of the crude oils relative to precipitation of asphaltenes was analyzed by titration with petroleum ether; the flocculation point was detected by optical microscopy.
Chromatographic/mass spectrometric analysis of the products and the original crude oil was performed on a DFS system (Thermo Electron, Germany). The ionization method was electron impact; the ionizing electron energy was 70 eV. We used an ID-BP5X capillary column (equivalent to DB-5MS) of length 50 m, diameter 0.32 mm. The carrier gas was helium, with flow rate 2 mL/min. The injector temperature was 250°C. Temperature programming conditions: from 60°C (isothermal for 1 minute) to 280°C with temperature rise rate of 10 degrees/minute, holding at the final temperature (total time for running a single sample, 60 minutes). Before injection into the instrument, the test sample was diluted in chromatography grade carbon tetrachloride in a concentration of ~10–3 g/μL. The program Xcalibur was used to process the mass spectral data. We recorded the total ion current mass chromatogram, followed by selected ion reconstruction and interpretation for m/z = 71 (alkanes), 82 (n-alkylcylohexanes), 92 (n-alkylbenzenes). In plotting the graphs for the hydrocarbon distribution, the area under the peak for the considered hydrocarbon was assigned to the total area for all the peaks present in the mass chromatograms for the specific ion.
The analysis results for the constituent composition of the original crude oil and the aquathermolysis products are given in Table 1. The increase in oil content and the decrease in resin content in aquathermolysis is due to degradation of the high molecular weight components [7–10]. In the composition of the catalytic aquathermolysis products, compared with the original crude oil we see a significant increase in the oil content and a decrease in the resin content by almost a factor of 2. These changes are reflected in the decrease in the density of the crude oil, from 960 to 953,6 kg/m3. Note that the decrease in the density cannot be due to dilution of the crude oil with tetralin or its dehydrogenation product naphthalene; the densities of both tetralin and naphthalene (respectively 973 and 1140 kg/m3) exceed the density of the original crude oil. The density of the crude oil, containing 5% tetralin, is equal to 966.7 kg/m3.
We should note the substantial increase in the asphaltene content (and the small increase in the density of the crude oil) in the noncatalytic process, and the insignificant increase in the catalytic process. Kaolin clay has acid properties, which promotes both cracking and polymerization reactions, in the absence of a source of hydrogen in the system leading to formation of high molecular weight products. The catalyst promotes reactions with hydrogen transfer from tetralin and naphthene/aromatic components of the crude oil, having hydrogen donor properties, to free radicals and thereby ensures their saturation and prevents recombination [32]. Reactions of hydrogenolysis of carbon–heteroatom bonds, hydrogenation of aromatic rings, and partial degradation of C–C bonds in resin and asphaltene molecules are also intensified [20, 21].
The results of fractional distillation at atmospheric pressure of the original crude oil and the aquathermolysis products (Fig. 1) show that treatment of heavy oils with a catalyst and a proton donor in a steam environment leads to de novo formation of light fractions which were absent in the original crude oil. Thus the yield of the 70°C-120°C fraction is 12.2%, the yield of the 70°C-200°C fraction increases by almost a factor of 6, the yield of the 70°C-250°C fraction increases from 6% to 29.5%. Therefore the crude oil, due to de novo formation of hydrocarbons [6], is enriched in the gasoline and kerosene fractions.
According to the results of the rheological studies, the viscosity of the crude oil at 5°C with catalytic conversion is reduced from 11.8 to 0.186 Pa·s for shear rate 1 s-1, and from 6.3 to 0.07 Pa·s for shear rate 320 s-1, i.e., by 98%. The degree of reduction in the viscosity achieved is quite consistent with the results of catalytic aquathermolysis in the presence of a proton donor that have been obtained by other researchers (86.7% in [21], 99.3% in [33]). Such a significant reduction in viscosity is due to the decrease in resin content by a factor of 1.7, the increase in the content of light fractions, oils, and also weakening of intermolecular interactions of aggregate combinations because of the appearance of tetralin/naphthalene in the system, improving the dissolving power of the dispersion medium and dispersing the asphaltene aggregates [34].
The IR spectra of the asphaltenes are shown in Fig. 2, and the calculated spectral coefficients are given in Table 2. For hydrothermal conversions, we see an increase in the aromaticity of the crude oil and a decrease in the aliphaticity. The aromaticity of the asphaltenes significantly increases as a result of reactions of degradation of the aliphatic substituents and condensation of aromatic rings under radical recombination conditions. For the aquathermolysis process, there is typically an increase in the degree of oxidation in the crude oil and the asphaltenes because of hydrolysis of the carbon–heteroatom bonds with formation in particular of phenols and alcohols [17, 18, 20, 35]. The increase in the degree of oxidation is also due to running the experiments in the presence of air, which implies occurrence of oxidative cracking processes at high temperatures. For noncatalytic aquathermolysis, the marked increase in the degree of sulfurization, characterizing the content of –SO groups, is the reason for oxidation of sulfides to form sulfoxides. The degree of branching of the asphaltenes remains practically unchanged, but the aliphaticity decreases due to degradation of side chains. In the catalytic aquathermolysis products, compared with the original crude oil, the content of bicyclic aromatic structures (D 865-880 + D 745-770) increases [36] in connection with de novo formation of naphthalene from tetralin.
In analyzing the spectra of the asphaltenes, we note the appearance with aquathermolysis of absorption bands in the region 1701–1709 cm–1, corresponding to stretching vibrations of the carbonyl group conjugated with aromatic structures [36]. The possibility of formation of acids, ketones, aldehydes with high-temperature conversions of organic compounds in the presence of water was demonstrated in [17].
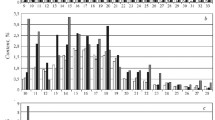
The distributions of n-alkanes, n-alkylcyclohexanes, and n-alkylbenzenes in the original crude oil and in the aquathermolysis products, determined by chromatography/mass spectrometry, are shown in Fig. 3 a-c. As we see, for noncatalytic aquathermolysis, generation of low molecular weight C13–C18 homologs occurs; the maximum of the distribution, as in the original crude oil, is found at C18. For catalytic aquathermolysis, we observe even more marked de novo formation of C11–C17 n-alkanes along with C20–C30 alkanes, the distribution of which is different from their distribution in the original crude oil. The ratio of the n-alkane contents (C11–C20)/(C21– C34) for the original crude oil is equal to 3.43; during noncatalytic and catalytic aquathermolysis, the ratio increases respectively to 5.68 and 4.71. While compounds with an odd number of carbons predominate in the composition of the C19–C28 n-alkanes of the original crude oil, in the catalytic aquathermolysis products C19–C28 n-alkanes with even and odd numbers of carbon atoms are uniformly distributed. The odd/even ratio for C11–C34 n-alkanes of the original crude oil is equal to 0.8 and does not change with noncatalytic aquathermolysis, but increases to 0.87 for catalytic conversion.
Distribution of n-alkanes (a) [bands 17 and 18 reflect the total content of respectively C17
n-alkane and pristane, C18
n-alkane and phytane], monoalkylcyclohexanes (b), and monoalkylbenzenes (c) in the original crude oil and in the aquathermolysis products: ■ represents the original crude oil;  ,
,  represent respectively noncatalytic and catalytic aquathermolysis.
represent respectively noncatalytic and catalytic aquathermolysis.
In aquathermolysis, the content of C12–C17, C19–C21 n-alkylcyclohexanes increases; the maximum of the distribution does not change. The distribution of n-alkylbenzenes in the original crude oil and the noncatalytic conversion product is bimodal with maxima at C14 and C20, while in the catalytic aquathermolysis product the n-alkylbenzenes are unimodally distributed with maximum at C14. The increase in the content of C12, C16, C18 n-alkylbenzenes by about a factor of 5 in catalytic conversion suggests the presence of the indicated hydrocarbons in bound form within the resin and asphaltene molecules [14]. Rapid generation of almost all the considered n-alkylbenzenes is due to occurrence, in the presence of the hydrogenolysis catalyst and tetralin, of processes of degradation of methylene and heteroatomic “bridges” in the resins and resin-like asphaltenes of the “archipelago” type, linking individual aromatic fragments of the molecules in these compounds. De novo formation of n-alkanes, on the other hand, is due to detachment of side chains from the asphaltene molecules of the “continents”, containing a single aromatic “flat” structure.
The average molecular weight of asphaltenes in the original crude oil, determined from MALDI mass spectra (Fig. 4), is equal to 1650; for the asphaltenes in the noncatalytic and catalytic aquathermolysis products, it is respectively 1720 and 1420. The small increase in molecular weight in the case of noncatalytic conversion of the crude oil is possibly due to rapid occurrence of the reactions of oxidation and polymerization in acid medium (the kaolin clay) when oxygen is present in the reaction system, in the absence of a proton donor, which is consistent with the data in [37]. The decrease in the average molecular weight of the asphaltenes during catalytic aquathermolysis is explained by degradation of the alkyl substituents, and correlates with the increase in aromaticity detected from IR spectroscopy data.
From the EPR spectra of the asphaltenes, it follows that as a result of hydrothermal processes, the concentration of VO-porphyrins decreases due to their thermal degradation (Table 3). In the catalytic and noncatalytic process, we observe a significant increase in the concentration of free radicals due to occurrence of reactions of degradation and oxidative conversions.
The stability of the crude oil to flocculation of asphaltenes changes little in the noncatalytic hydrothermal process: from 3.56 mL/g for the original crude oil up to 3.66 mL/g, which is possibly due to the presence of finely dispersed clay in the asphaltenes, preventing their aggregation. At the same time, incatalytic aquathermolysis, the stability is reduced down to 1.42 mL/g, which is due to the increase in the asphaltene/resin ratio from 0.16 for the original crude oil to 0.29 for the catalytic conversion product, the significant increase in the aromaticity of the asphaltenes and the content of paramagnetic centers in them.
In the elemental composition of the crude oil, after the experiments the atomic ratio H/Cat decreases (respectively 1.75, 1.68, and 1.68 for the original crude oil and the noncatalytic and catalytic aquathermolysis products), the oxygen content increases (respectively 3.93, 6.12, and 7.64 wt.%), which correlates with IR spectroscopy data and confirms the increase in the aromaticity of the crude oil and the content of oxygen-containing structures (high-temperature hydrolysis products). A decrease in the H/Cat ratio is also typical for the asphaltenes (1.12, 1.06, and 1.03 respectively), the sulfur content decreases (6.75, 4.7, and 6.2 wt.%), the nitrogen content decreases (1.54, 1.08, and 1.38 wt.%), and the oxygen content increases.
According to x-ray fluorescence analysis data, after catalytic aquathermolysis the rock contains Co and Ni, which are absent in the original kaolin clay. This suggests adsorption of the metals from the catalyst on the rock. In the crude oil and the asphaltenes, after the experiment there is no cobalt but the nickel content is somewhat increased. The fact that the catalyst is adsorbed on the rock (probably in the form of metal sulfides, since the sulfur content in the rock also increases) is important for commercial implementation of the process of in situ upgrading of heavy oils and natural bitumens. Adsorption of metals on rock will make it possible to reduce the amount of catalyst used and to realize the concept of heterogeneous catalysis in situ, used in the CAPRI technology [38], without placing a heterogeneous catalyst around the production wells.
According to the thermal analysis results, the original rock does not contain organic matter. The organic matter content in the rock removed from the reactor, after extraction with benzene, is 2% for noncatalytic aquathermolysis and 1% for catalytic aquathermolysis. The ratio of the content of the 20°C-400°C fraction to the 400°C-500°C fraction in the first case is equal to 0.1; in the second case, 0.3. Obviously in the absence of a catalyst and a proton donor in the reaction mixture, more coke-like residue is formed, which is concentrated in the rock, while hydrogen transfer from the naphthene/aromatic component in the presence of transition metals efficiently inhibits coke formation.
Chromatographic analysis of the gases sampled when the autoclave was opened revealed the presence of methane and carbon dioxide: the product of degradation of oxygen-containing compounds formed upon hydrolysis [18]. We note that formation of CO2 (content in the sample of gases from catalytic aquathermolysis: 14.69%) has a favorable effect on the process of oil recovery due to the fact that it dissolves in the crude oil and improves its mobility. When the autoclave was opened, we also noted a strong odor of hydrogen sulfide.
The studies presented allow us to suggest that a proton donor and a catalyst favor occurrence of the reactions of hydrogenolysis and hydrogenation of resin/asphaltene compounds, with de novo formation of light fractions, hydrocarbon and non-hydrocarbon gases. The aqueous phase enables hydrolysis of the heteroatomic compounds, and also, as a result of the high dissolving power at a temperature of 300°C, acts as a diluent, reducing the concentration of free radicals in the reaction mixture and preventing their recombination. Further research in the area of catalytic aquathermolysis is of interest, and may be aimed at optimization of the concentrations and searching for more accessible proton donors and catalysts.
References
S. K. Maity, J. Ancheyta, and G. Marroquin, Energy & Fuels, 24, 2809-2816 (2010).
J. B. Hyne, J. W. Greidanus, J. D. Tyrer et al., in: Second International Conference on The Future of Heavy Crude and Tar Sands, Caracas, Venezuela, 7-17 February 1982, McGraw Hill, New York (1984), pp. 404-411.
Y. Takeuchi, D. Miyamoto, A. Kishita et al., in: Petroleum Society’s Eighth Canadian International Petroleum Conference (Fifty-Eighth Annual Technical Meeting), 12-14 June 2007, Calgary, Alberta, Canada.
Li-Qun Zhao, Zhen-Min Cheng, Yong Ding et al., Energy & Fuels, 20, 2067-2071 (2006).
A. M. Kiyamova, G. P. Kayukova, and G. V. Romanov, Neftekhimiya, 51, No. 4, 243-253 (2011).
G. P. Kayukova, G. V. Romanov, R. G. Luk’yanova et al., Organic Geochemistry of Sedimentary Rock Formation and Bedding in the Tatarstan Region [in Russian], GEOS, Moscow (2009). 487 pp.
A. M Kiyamova, G. P. Kayukova, V. I. Morozov et al., Technologii Nefti i Gaza, No. 1, 40-47 (2007).
G. P. Kayukova, L. Z. Nigmedzyanova, A. G. Romanov et al., Neftekhimiya, 45, No. 4, 252-261 (2005).
G. P. Kayukova, A. M. Kiyamova, L. Z. Nigmedzyanova et al., Neftyanoe Khozyaistvo, No. 2, 105-109 (2007).
G. P. Kayukova, A. M. Kiyamova, L. Z. Nigmedzyanova et al., Neftekhimiya, 47, No. 5, 349-361 (2007).
V. R. Antipenko, Izvestiya Tomskogo Politekhnicheskogo Universiteta, 319, No. 3, 125-129 (2011).
V. R. Antipenko and O. A. Golubina, Izvestiya Tomskogo Politekhnicheskogo Universiteta, 309, No. 2, 174-179 (2006).
V. R. Antipenko, O. A. Golubina, I. V. Goncharov et al., Izvestiya Tomskogo Politekhnicheskogo Universiteta, 308, No. 6, 122-127 (2005).
V. R. Antipenko, Thermal Transformations of High-Sulfur Natural Asphaltite: Geochemical and Technological Aspects [in Russian], Nauka, Novosibirsk (2013). 184 pp.
N. N. Rokosova, Yu. V. Rokosov, S. I. Uskov et al., Neftekhimiya, 41, No. 4, 243-257 (2001).
A. Kruse and E. Dinjus, J. of Supercritical Fluids, 39, 362-380 (2007).
A. R. Katritzky, D. A. Nichols, M. Siskin et al., Chem. Rev., 101, No. 4, 837-892 (2001).
A. R. Katritzky and S. M. Allin, Accts. Chem. Res., 29, 399-406 (1996).
P. D. Clark, J. B. Hyne, and J. D. Tyrer, Fuel, 63, 125-128 (1984).
V. A. Lyubimenko, N. N. Petrukhina, B. P. Tumanyan et al., Khim. Tekh. Topliv Masel, No. 4, 27-33 (2012).
Yongjian Liu and Hongfu Fan, Energy & Fuels, 16, 842-846 (2002).
R. Hasemi and P. Pereira-Almao, in: Canadian Unconventional Resources Conference, 15-17 November 2011, Calgary.
H. Fan and Y. Liu, Oil & Gas Journal, 100, No. 11, 60-62 (2002).
Fajun Zhao,Yongjian Liu, Yongbin Wu, Xin Zhao, and Longri Tan, Khim. Tekh. Topliv Masel, No. 4, 16-21 (2012).
S. Wen, Y. Zhao, Y. Liu et al., 2007 SPE International Symposium on Oilfield Chemistry, 28 February-2 March 2007, Houston; SPE 106180.
S. Jiang, X. Liu, and L. Zhong, SPE International Symposium on Oilfied Chemistry, 2-4 February 2005, Houston; SPE 91973.
Y. Chen, Y. Wang, C. Wu et al., Energy & Fuels, 22, 1502-1508 (2008).
K. Chao, Y. Chen, H. Liu et al., Energy & Fuels, 26, 1152-1159 (2012).
H. Fan, Y. Zhang, and Y. Lin, Fuel, 83, 2035-2039 (2004).
G. P. Kayukova, I. M. Abdrafikova, I. R. Sakhibgareev et al., Tekhnologii Nefti i Gaza, No. 5, 43-48 (2012).
M. Siskin and G. Brons, Energy & Fuels, 4, No. 5, 482-488 (1990).
K. A. Gould and I. A. Wiehe, Energy & Fuels, 21, 1199-1204 (2007).
Y. Chen, C. Yang, and Y. Wang, J. of Analytical and Applied Pyrolysis, 89, 159-165 (2010).
B. P. Tumanyan, Scientific and Applied Aspects of the Theory of Petroleum Dispersed Systems [in Russian], Tekhnika, Moscow (2000). 336 pp.
M. Siskin, G. Brons, and S. N. Vaughn, Energy & Fuels, 4, 488-492 (1990).
A. G. Sokolova, N. K. Nadirov, and A.E. Chebotarevskii, Infrared Spectra of Crude Oils and Natural Bitumens of the Caspian Region Basin [in Russian], GANG, Moscow (1997). 182 pp.
Yufeng Yi, Shuyuan Li, Fuchen Ding, and Hang Yu, Petroleum Science, No. 6, 194-200 (2009).
R. G. Moore, S. A. Mehta, J. D. M. Belgrave et al., in: Forty-Seventh Annual Technical Meeting of the Petroleum Society in Calgary, Alberta, Canada, 10-12 June 1996.
We would like to thank Yu. M. Ganeeva (Doctor of Chemical Sciences, Petroleum Chemistry and Geochemistry Laboratory) for carrying out the experiments on thermal analysis of the rocks, and also V. M. Babaev (Candidate of Chemical Sciences), O. B. Bazanova, E. A. Stroiteleva, and V. I. Morozov for assistance in carrying out the analytical studies of the aquathermolysis products performed at the Analytical Chemistry Center of the A. E. Arbuzov Institute of Organic and Physical Chemistry, Kazan Science Center of the Russian Academy of Sciences.
This work was funded by a subsidy provided as part of government support for Kazan Federal University to make it more competitive with leading international scientific and educational centers.
This work was funded by a subsidy provided to Kazan Federal University as part of a government mission to promote scientific research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 30 – 37, July – August, 2014.
Rights and permissions
About this article
Cite this article
Petrukhina, N.N., Kayukova, G.P., Romanov, G.V. et al. Conversion Processes for High-Viscosity Heavy Crude Oil in Catalytic and Noncatalytic Aquathermolysis. Chem Technol Fuels Oils 50, 315–326 (2014). https://doi.org/10.1007/s10553-014-0528-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-014-0528-y