Carbon adsorbents with mechanical strengths of 90-93% and a micropore volume of 0.16-0.3 cm3/cm3 have been obtained from nitrated asphaltites by the existing industrial technology. Compared to industrial carbon with the trademark KAD-iodine, medium-charred specimens are highly selective in extracting gold and silver from multicomponent polymetal solutions and have a high capacity for sorbing arsenic from waste waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Previous studies [1–3] have shown that it is possible to obtain high-quality carbon adsorbents by adding chemically modified asphaltenes to the charge with or without coal. Continuing these studies, we examined the use of nitrated asphaltenes for this purpose. The nitration technology that was employed is well-known in industry.
We used asphaltite obtained by deasphalting the tar of Arlansk oil with benzene. The asphaltite had the following characteristics: density 1154 kg/m3; softening point 178°C; content (wt. %): oils - 12.6; resins - 8; asphaltenes - 79.4.
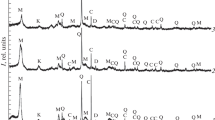
Nitration was carried out over 5 h with the use of concentrated nitric acid at 75-78°C. The product was washed to neutral reaction and then dried. A spectroscopic analysis performed on a UR-20 spectrometer revealed the presence of functional groups in the product. The analysis was performed using tablets pressed with potassium bromide. The most intensive band on the IR-spectra, in the1700 cm−1 region, corresponds to the carbonyl group; the bands in the 1545 and 1640 cm−1 regions correspond to C – NO2 groups. The spectra also contain absorption bands corresponding to the = CH2 and –CH3 groups; the lower-intensity bands in the 1640 cm−1 region correspond to aromatic nitrogen-bearing compounds, while the lower-intensity bands in the 850 cm−1 region correspond to aliphatic nitrogen-bearing compounds. The spectra also contain all of the bands that correspond to the ester and alcohol groups, since two processes - oxidation and nitration - take place in the reaction with nitric acid.
To obtain adsorbents, we used a product containing 6.8% nitrogen and 23.4% of a gel-fraction. Intensive gel formation takes place during carbonization of nitrated asphaltenes. Thus, part of the nitrated product was replaced by the initial asphaltite.
The adsorbents were obtained from a charge of the following composition (wt. %): nitrated asphaltite - 30; asphaltite - 14; coal dust - 20. We used coal of grade T from the Prokopevsk deposit (the Central mine). The charge was formed with the addition of 36 wt. % wood resin. The charge materials were mixed in a mixer at 60-70°C and shaped by pressing them through dies with cells 2-2.5 mm in diameter. The resulting granules were held at room temperature for 4-6 h and then dried in a rotary electric furnace at 100°C. The furnace drying operation was performed in an atmosphere of carbon dioxide for a period of 30 min. The carbonization process was carried out in the same furnace and same atmosphere in two stages: the granules were heated from 100 to 450°C in the first stage and from 450 to 800°C in the second stage. Total carbonization time was 40 min. The yield of carbonized product was 46-48%. Primary activation of the adsorbents together with complete removal of the volatile matter was done in the same furnace in a carbon-dioxide atmosphere at 800°C over a period of 40 min. Further activation to the medium-charred state was done in a steam atmosphere at 800-820°C.
The roentgenographic characteristics of the adsorbents and carbonized products were studied on a DRON-1 diffractometer. The x-ray photographs were taken with projection of the focus of the copper anode of the VSV-9 x-ray tube on a blazed diffraction grating. The photographs were obtained in the following regime: accelerating voltage 30 kV; current 20 mA. The beam of x-rays was formed using Soller slits. The primary structure of the packets of granules of the carbonized material corresponds to the presence of 2-3 plane atomic networks in one packet of structural elements. The packets do not have a three-dimensional structure or dense-packed longitudinal chains, i.e. the carbonized materials contain degradative elements. Table 1 shows the roentgenographic characteristics of one of these materials and a medium-charred adsorbent.
Activation of the carbonated materials significantly alters their structure: density increases, the distance between layers decreases, and the size of the packets increases to 4-5 plane atomic networks. The increase in packet size is due to ordering and aromatization of the material. There is also a change in nitrogen content relative to the original material. For example, adsorbent AN-27 contains 2% nitrogen. High-temperature activation is apparently accompanied by the degradation of oxygen- and nitrogen-bearing compounds that were stable at the carbonization temperature and prevented ordering of the carbonized materials’ structure.
Table 2 shows characteristics of the pore structure of the carbonized materials and adsorbents. The changes seen in the molecular structure with activation are related to the formation of this structure. An increase in charring is accompanied by an increase in total pore volume. The pore volume that is accessible to the pycnometric liquids in the series CH3OH < C6H6 < CCl4 decreases with an increase in the critical dimensions of their molecules, which is an indication of the nonuniformity of the pore structure (ultra-microporosity).
To explore the possible areas of application of the adsorbents that were obtained, we studied their sorptivity and selectivity with respect to noble metals and arsenic in waste water.Footnote 1 Table 3 shows the kinetics of sorption and selectivity with respect to gold and silver. The kinetic characteristics of the sorption of gold and silver were obtained by a method based on a constant concentration of the target component and a constant saline composition (with replacement of the solution) under static conditions with agitation in the mixer. The concentrations of the metals were determined by atomic-absorption spectrophotometry on a Perkin-Elmer 503 spectrophotometer. The mass ratio of sorbent to solution was 1:2000. The selectivity coefficient was determined by the method described in [4].
Selectivity with respect to gold was determined with the use of a solution of the following composition (mg/liter): gold - 0.9; silver - 0.76; copper - 43.1; zinc - 37.7; nickel - 0.66; cobalt - 0.29; cyanide ions - 109; calcium oxide - 0.002.
Selectivity with respect to silver was determined by using solutions from which some gold had been previously been removed, since the adsorbents were saturated with dicyanoaurates earlier than with dicyanoargentates. A solution having the following composition (mg/liter) was used: gold - 0.02; silver - 0.75; copper - 18.28; zinc - 25.89; nickel - 0.17; cobalt - 0.16; cyanide ions - 0.012; calcium oxide - 0.006.
Commercial KAD-iodide coal was used as the material of the control specimen. It can be seen from Table 3 that, beginning with a charring of 22%, the sorptivity of the test specimens with respect to gold becomes greater than that of the control specimen. The selectivity of sorbent AN-27 is twice as great as that of the KAD-iodide sorbent. A similar pattern is seen for sorptivity and selectivity with respect to silver. Thus, specimens that have undergone a moderate degree of activation are best-suited for the selective extraction of noble metals from multicomponent polymetal solutions.
Waste water discharged from a roasting plant and having an average arsenic content of 2.57 mg/liter was used to study sorption activity with respect to arsenic. Arsenic content was determined by the photometric method with the use of silver diethyldithiocarbamate (Table 4).
The ratio of the mass of sorbent to the mass of the solution was 1:1000. It can be seen that all of the investigated specimens had roughly the same sorptivity after 100 h of sorption. However, preference should be given to the low-charred specimens (4-10% charring) based on the rate at which arsenic is sorbed.
The study of nitrated asphaltenes as a feedstock for the production of adsorbents showed that it is possible to obtain specimens which have a higher degree of selectivity with respect to gold and silver than do coal-based adsorbents. However, the complexity of the nitrating operation - high temperatures, corrosionally aggressive media, the need for long washing of the products to neutral reaction, and the formation of acidic wash water - makes it preferable to use polycondensation products for adsorbent production [5].
Notes
The study of sorptivity with respect to noble metals and arsenic was performed by A. I. Gorbovskii in the laboratory at the Kochkanarsky plant.
References
Yu. V. Pokonova, Highly Effective Carbon Adsorbents from Petroleum Residues [in Russian], TsNIITEneftekhim, Moscow (1985).
Yu. V. Pokonova, Petroleum Residues [in Russian], Rikon, St. Petersburg (2008).
Yu. V. Pokonova, Carbon, 29, No. 7, 865-869 (1991).
Yu. V. Pokonova and A. I. Grabovskii, Khim. Tverd. Tela, No. 7, 91-95 (1988).
Yu. V. Pokonova, Fuel Sci. and Technol. Int. L., 9, No. 10, 1199-1210 (1991).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 38 – 40, July – August, 2012.
Rights and permissions
About this article
Cite this article
Pokonova, Y.V. Producing adsorbents from nitrated asphaltites. Chem Technol Fuels Oils 48, 308–312 (2012). https://doi.org/10.1007/s10553-012-0373-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-012-0373-9