Abstract
Purpose
Epidemiological studies have suggested a protective effect of dietary fiber intake on breast cancer risk while the results have been inconsistent. Our study aimed to investigate the association between dietary fiber intake and breast cancer risk and to explore whether this association is modified by reproductive factors and hormone receptor status of the tumor.
Methods
A total of 44,444 women aged 45 to 74 years from the Japan Public Health Center-based Prospective Study were included in analyses. Dietary intake assessment was performed using a validated 138-item food frequency questionnaire (FFQ). Hazard ratios (HRs) and 95% confidence intervals (CIs) for breast cancer incidence were calculated by multivariate Cox proportional hazards regression models.
Results
During 624,423 person-years of follow-up period, 681 breast cancer cases were identified. After adjusting for major confounders for breast cancer risk, inverse trends were observed but statistically non-significant. Extremely high intake of fiber was associated with decreased risk of breast cancer but this should be interpreted with caution due to limited statistical power. In stratified analyses by menopausal and hormone receptor status, null associations were observed except for ER-PR- status.
Conclusions
Our findings suggest that extreme high fiber intake may be associated with decreased risk of breast cancer but the level of dietary fiber intake among Japanese population might not be sufficient to examine the association between dietary fiber intake and breast cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequently diagnosed cancer among females in both developing and developed countries with an estimated 1.67 million new cases diagnosed in 2012 [1]. It is also the most common cancer among Japanese women with a predicted 72,500 newly diagnosed cases in 2011 [2]. Dissemination of breast cancer screening and changes in dietary, hormonal, and reproductive factors [3, 4] have rapidly increased the incidence in most regions of the world [5]. Wide international disparity in breast cancer incidence [6] and its different patterns among immigrants [7] indicate the importance of not only the genetic factors but also modifiable risk factors including dietary factors in the etiology of breast cancer.
Dietary fiber has been hypothesized to reduce the risk of breast cancer over three decades ago [8]. Soluble fiber is contained in oats, legumes and seaweeds, and could modulate insulin resistance and insulin-like growth factors, the increased concentrations of which have been associated with an increased breast cancer risk [9]. Insoluble fiber is, on the other hand, found in seeds and whole grains, and may help to inhibit intestinal reabsorption of estrogens and thus increase fecal excretion of estrogen. While prolonged exposure to estrogen can increase the risk of breast cancer, insoluble fiber may lower the level of circulating estrogen concentrations [10]. A meta-analysis of 16 prospective studies reported an inverse association between soluble fiber and breast cancer risk but not insoluble fiber, fruit fiber, vegetable fiber, or cereal fiber [11]. The findings from more recent studies found that vegetable fiber was associated with a decreased risk of breast cancer but not with other types of fiber [12, 13].
The previous JPHC Study has investigated the association between fiber-containing food items such as vegetables, fruits, and soyfoods including miso soup and breast cancer risk. While miso soup and isoflavone intake was significantly and inversely associated [14], and fruits and citrus fruits consumption with borderline significance [15], there has not been a study with total dietary fiber intake. Furthermore, fiber consumption in Japan is lower than that of Western populations [16], which highlights the importance of the study in Japan. Therefore, we explored the potential association between dietary fiber intake and risk of breast cancer in the Japanese population, and investigated whether the association is influenced by reproductive factors and hormone receptor status.
Materials and methods
Study population
The Japan Public Health Center-based Prospective Study (JPHC Study) is an ongoing cohort study designed to assess the association between lifestyle factors and cancer and cardiovascular disease in the Japanese population. The study was commenced in 1990 for Cohort I (n = 61,595) and 1993 for Cohort II (n = 78,825) with 140,420 participants (68,772 men and 71,698 women) residing in municipalities supervised by 11 public health centers across Japan. Details of the JPHC Study methodology have been described in a previous study [17]. The study protocol was approved by an institutional review board of the National Cancer Center, Tokyo, Japan (approval number: 13–021) and The University of Tokyo (approval number: 10508).
The study population consisted of women aged 40 to 59 years in Cohort I and 40 to 69 years in Cohort II. Participants from two public health center areas (n = 12,515) were not included in the study due to unavailability of complete data on cancer incidence. The self-administered 5-year follow-up questionnaire was sent to subjects aged 45 to 74 years in 1995 and 1998 for Cohort I and Cohort II, respectively, and used as the starting point of this study. After excluding 208 ineligible participants and a total of 3,525 participants who had died, moved, or were lost to follow-up, the remaining 55,450 persons were eligible for participation. Of those, 48,279 returned the 5-year questionnaire (response rate 87.1%), and 3,835 women were further excluded due to past history of cancer, extreme caloric intake (lower and upper 2.0% of the distribution), or missing data on dietary fiber. The final analytical cohort of 44,444 women was included in the multivariable analyses.
Exposure measurement
Dietary information used in the present study was obtained through the common 5-year follow-up survey, in which the dietary assessment was carried out using a validated self-administered food frequency questionnaire (FFQ) [18]. Participants were asked to report average consumption frequency and portion size of 138 food and beverage items consumed in the past year. The consumption of seasonal fruits and vegetables (grams/day) were also calculated by asking the frequency of intake and considering length of each season [19].
The consumption of daily nutritional intake was calculated in reference to the Standard Tables of Food Composition in Japan (5th revised and enlarged ed.) and the amount of dietary fiber contained in each food item (grams/day) was estimated by the modified Prosky method, which separately quantifies soluble and insoluble fiber in food by imitating the bodily digestive system [20]. All of the nutritional covariates, except for alcohol, were adjusted for total energy intake using the residual method [21]. The validity and reproducibility of the FFQ for dietary assessment were evaluated and marked Spearman’s correlation coefficients of 0.48 for total fiber, 0.53 for soluble fiber, and 0.46 for insoluble fiber [22], which are comparable to previous studies [23, 24]. In addition to analyses by fiber category, two commonly consumed food items were added as exposure variables, namely natto/fermented soybean and rice. Natto and rice were the highest contributors to the daily intake of soluble and insoluble fiber, accounting for 4% and 11%, respectively, in this study population [22]. Natto and rice each comprised 3% and 8% of total fiber intake, and other major sources also included miso/fermented soybean baste (5%), carrots (3%), apples (3%), and cabbage (3%). Information on other covariates, such as anthropometric measurements, reproductive, and lifestyle-related factors was acquired using the self-reported questionnaire in the 5-year follow-up survey.
Ascertainment of breast cancer cases and follow-up of the cohort
Incidence of breast cancer was identified via active patient notification from major local hospitals in each study area and record linkage with a population-based cancer registry. Breast cancer cases were classified using codes C500-509 of the International Classification of Diseases for Oncology (3rd ed.) [25]. Death certificates were used to ascertain 11 cases (Death Certificate Notification; DCN: 2.0% of total cases), of which information on diagnosis was unavailable for 9 cases (Death Certificate Only; DCO: 1.6%). Ninety-seven per cent of all the cases diagnosed during the follow-up period were verified microscopically. Estrogen receptor (ER) and progesterone receptor (PR) status were defined by either immunohistochemistry or enzyme-linked immunosorbent assay.
Participants were censored on the date of breast cancer diagnosis, migration from study area, death, or end of follow-up period (31 December, 2011) whichever came first. Person-years of follow-up were calculated for each participant from the 5-year follow-up survey until the date of censoring.
Statistical analysis
Participants were divided by quartiles according to their energy-adjusted intakes of each exposure variable, namely total fiber, soluble fiber, insoluble fiber, natto, and rice. The group with the highest consumption was indicated as Q4 and the lowest as Q1. Their baseline characteristics were compared between groups, using ANOVA or Pearson’s chi–squared test where appropriate. A Cox proportional hazards regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), with Q1 as the reference group. The proportional hazards assumptions were verified and satisfied with Schoenfeld residuals. All the statistical analyses in the present study were performed using Stata version 13.0 (StataCorp, College Station, Texas USA).
In the primary analyses, the multivariable models were adjusted for age, areas of public health centers, BMI at 5-year follow-up (< 18.5, 18.5–23.9, >23.9), age at menarche (≤ 13, 14, 15, ≥16 years), age at first birth (< 26, ≥26 years), parity (nulliparous, 1–2, 3, ≥4), age at menopause (pre-menopause, ≤44, 45–54, ≥55 years), use of exogenous female hormones (never, ever), smoking status (never: non-smokers, ever: past or current smokers), leisure-time physical activity (≤ 3 days/month, 1–2 days/week, ≥3 days/week), alcohol intake (regular drinker: >150 g of ethanol/week, non-regular drinker: ≤150 g of ethanol/week), total energy intake, and total energy-adjusted intakes of fat, isoflavones, carbohydrates, and vitamin C. Vitamin C was included in the model since high intake of vitamin C was associated with increased risk of breast cancer in our previous report [15]. Missing data were created for each covariate as an indicator variable. Further analysis explored whether extremely high intake of dietary fiber could modify the risk of breast cancer and was performed by dividing the highest consumption group (Q4) into tertiles.
In the secondary analyses, stratification by menopausal status (pre or post) and sub-group analysis by ER/PR status were further performed. All statistical tests were 2-sided, and p < 0.05 was considered statistically significant.
Results
During 624,423 person-years of follow-up (an average of 14.0 years), 681 cases of breast cancer were newly diagnosed. The median age of diagnosis was 65 years. Of which, 333 cases (48.9%) had information on ER status and 326 cases (47.9%) on PR status. Mean age at 5-year follow-up was 58.4 years and people with higher fiber consumption were slightly older. The amount of total fiber intake ranged from the median of 7.9 g/day in the lowest category to 18.1 g/day in the highest.
Table 1 shows the characteristics of the study participants by categories of dietary fiber intake. Participants who consumed more fiber were less likely to be current smokers, regular drinkers, and more likely to be physically active on a regular basis. They also had higher level of isoflavone, fat, vitamin C, and carbohydrate intakes.
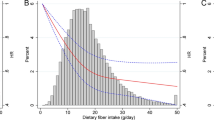
Table 2 shows the results of multivariate analyses. Overall, no significant association was detected between the consumption of fiber and breast cancer risk in this cohort after adjusting for all potential confounding factors (HR 0.78, 95% CI 0.55–1.09, comparing Q4 with Q1). Adjusting for total energy-adjusted intake of vitamin C and carbohydrates made an inverse trend with total fiber intake becoming evident but non-significant. These results did not substantially change in the analysis by dose or with non-smokers (results not shown). In the subtertile analysis, extremely high intake of dietary fiber was significantly associated with decreased risk of breast cancer. HRs for the highest tertile of total fiber were 0.63 (95% CI 0.40–0.98) for the highest category (Sub3); 0.68 (95% CI 0.45–1.04 for the middle category (Sub2); and 0.93 (95% CI 0.64–1.34) for the lowest category (Sub1), with p trend of 0.04 and the lowest quartile (Q1) as the referent group (Fig. 1).
Hazard ratios (HRs) and 95% confidence intervals (CIs) for breast cancer risk within the highest quartile of total fiber intake. aThe reference group is the lowest quartile (Q1) of total fiber intake and a subtertile (Sub 1–3) is tertiles of the highest quartile (Q4). Numbers of breast cancer cases were 180 for Q1, 164 for Q2, 170 for Q3, 63 for Sub 1, 52 for Sub 2, and 52 for Sub 3
Table 3 reports the association of dietary fiber intake and risk of breast cancer by menopausal status. Among 41,501 women whose menopausal status was available, 31,931 of them were postmenopausal with 465 cases of breast cancer. There was an overall reduction in the risk of postmenopausal breast cancer but it was statistically non-significant.
In sub-group analysis by ER/PR status of breast tumors, information on both receptors was available in 323 (47.4% of all) cases, as presented in Table 4. Consumption of soluble fiber showed a significant positive association with ER−PR−breast cancer risk (HR 5.45, 95% CI 1.02–29.20, p trend: 0.03) but this should be interpreted with caution, as the number of cases was limited. Null associations were observed with the other types of hormone receptor-defined breast cancer.
Discussion
In the present population-based prospective study, extreme high intake of total fiber was associated with decreased risk of breast cancer. However, the findings with other types of dietary fiber showed inverse yet statistically non-significant trends. Given these results, the association between dietary fiber and breast cancer risk appears non-linear since only high level of consumption was significantly associated, but it should be interpreted with caution since the number of cases in the subtertile analysis was small. The overall null associations in the present study might be explained by small ranges of fiber consumption between quartiles. In a meta-analysis of 16 prospective cohort studies [11], the mean fiber intake of the highest category in an Asian cohort was the lowest in comparison to the rest of the cohorts in European and American countries (16.3, 29.9, and 25.7 g/day, respectively). Indeed, the inverse association was only evident among studies with high levels (≥25 g/day) or large ranges (≥13 g/day) of fiber consumption. Moreover, regional difference in sources of dietary fiber may be also noted as cereal, vegetables, and fruits were the main contributors in European countries [12, 13].
The overall risk of postmenopausal breast cancer was non-significantly lower than that of pre-menopausal breast cancer and the observation was consistent with some of the previous studies, which found an inverse association with postmenopausal cancer [26, 27]. Dietary fiber consumption may indirectly reduce the risk of postmenopausal breast cancer by preventing obesity and Type 2 diabetes through insulin resistance control [28, 29]. In the subanalysis by ER/PR status, the risk of ER-PR- breast cancer was significantly elevated with consumption of soluble fiber. However, previous studies found a strong inverse association with ER-PR- breast tumor [13, 30]. The interpretation of this result should be made with caution due to limited statistical power. Epidemiological studies have also suggested that factors related to estrogen metabolism are more strongly associated with ER+ tumors, while ER- tumors may be more susceptible to non-hormonal exposures such as diet [13, 30]. Further research may be needed to explore the association in the Japanese population to investigate how dietary fiber could play a role in carcinogenesis of both ER+ and ER− tumors.
Moreover, high intakes of citrus fruit [15] and carbohydrate [31] are observed to have an increased risk of breast cancer in previous studies. Although a null association was observed, participants with higher level of dietary fiber intake had higher vitamin C and carbohydrate intake in this study and thus the possibility of diluted effect of fiber in our data may not be eliminated. The latest report from the World Cancer Research Fund suggested a reduction in the breast cancer mortality with consumption of high-fiber foods [31], whilst the association with its incidence has yet been inconclusive [32]. Further research is required to examine the association between a wider range of dietary fiber intake and breast cancer mortality and recurrence.
There are a few limitations in the present study. First, the self-reported questionnaire would have introduced to a measurement bias. The FFQ may be a less accurate estimation of dietary intake when compared to a 24-hour recall method, and therefore has the potential to attenuate the estimated hazard ratios [33]. The correlation coefficients between the FFQ and 28-day (or 14-day) dietary records were rather low (0.48 for total fiber, 0.53 for soluble fiber, and 0.46 for insoluble fiber). However, the results are similar to previous reports from Japan [23, 24].
Second, there is a possibility of further confounding due to the effect of health-conscious behavior of people who had breast cancer screening. In Japan, biennial mammography screening in combination with a clinical breast examination is recommended for women aged 40 years or older since 2004 [34]. The health-conscious behavior could affect the dietary pattern and thus be a confounding factor. Nevertheless, the questionnaire asked whether a participant had mammography in the past year only and therefore was unable to capture the effect of biennial mammography screening.
Finally, information on ER/PR subtypes is available for 50% of the breast cancer cases in the present study. This was unavoidable since ER/PR status was not used to be regarded as substantial information for clinical treatment, and data prior to 2002 were attained by retrospective review of medical records or pathology reports [35]. In addition, some categories in the subanalyses by menopausal status and ER/PR status had small number of cases. Interpretation should be made with caution due to limited statistical power and possibility of chance finding.
Despite these limitations, to our knowledge, this is the first large prospective study to investigate the potential link between dietary fiber and risk of ER/PR-defined breast cancer in the Japanese population although overall associations were not significant. The incidence of breast cancer in Japan has been steeply increasing over the past few decades, but it is still lower than that of European or North American countries [36]. The incidence of breast cancer observed in this study is in accordance with that in Japan [37]. Major strengths of this study also include high response rate (87.1%) and long follow-up period with negligible number of subjects lost to follow-up (0.1%). Possibility of recall and selection bias has been kept minimal as information on exposure was collected before diagnosis of breast cancer. Moreover, incidence of breast cancer was confirmed by cancer registry, rather than self-reporting, to reduce the potential for misclassification of the outcome.
In conclusion, while previous studies have found protective effects of high dietary fiber consumption against breast cancer, our findings were unable to draw clear comparison due to lower intake of fiber among Japanese population. Future research is needed to further investigate the association in the Japanese or Asian population between high levels of dietary fiber intake and the potential effect on breast cancer survival and recurrence.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2014) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. doi:10.1002/ijc.29210
National Cancer Center (2016) Cancer Registry and Statistics. http://ganjoho.jp/reg_stat/statistics/stat/summary.html.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA (2005) Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol 34(2):405–412. doi:10.1093/ije/dyh414
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C et al (2013) Cancer incidence and mortality worldwide: IARC cancerbase no. 11. International Agency for Research on Cancer, Lyon
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. doi:10.1002/ijc.25516
Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63(6):963–966
Goldin BR, Adlercreutz H, Gorbach SL, Warram JH, Dwyer JT, Swenson L et al (1982) Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med 307(25):1542–1547. doi:10.1056/NEJM198212163072502
Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ (2000) Beneficial effects of high dietary fiber intake in patients with Type 2 diabetes mellitus. N Engl J Med 342(19):1392–1398. doi:10.1056/NEJM200005113421903
Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C et al (2005) Serum sex steroids in premenopausal women and breast cancer risk within the European prospective investigation into cancer and nutrition (EPIC). J Natl Cancer Inst 97(10):755–765. doi:10.1093/jnci/dji132
Aune D, Chan DS, Greenwood DC, Vieira AR, Rosenblatt DA, Vieira R et al (2012) Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol 23(6):1394–1402. doi:10.1093/annonc/mdr589
Deschasaux M, Zelek L, Pouchieu C, His M, Hercberg S, Galan P et al (2013) Prospective association between dietary fiber intake and breast cancer risk. PLoS ONE 8(11):e79718. doi:10.1371/journal.pone.0079718
Ferrari P, Rinaldi S, Jenab M, Lukanova A, Olsen A, Tjonneland A et al (2013) Dietary fiber intake and risk of hormonal receptor-defined breast cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr 97(2):344–353. doi:10.3945/ajcn.112.034025
Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S, for the Japan Public Health Center-Based Prospective Study on Cancer Cardiovascular Diseases Group (2003) Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 95(12):906–913. doi:10.1093/jnci/95.12.906
Suzuki R, Iwasaki M, Hara A, Inoue M, Sasazuki S, Sawada N et al (2013) Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective study. Cancer Causes Control 24(12):2117–2128. doi:10.1007/s10552-013-0289-7
Fukuda S, Saito H, Nakaji S, Yamada M, Ebine N, Tsushima E et al (2007) Pattern of dietary fiber intake among the Japanese general population. Eur J Clin Nutr 61(1):99–103
Tsugane S, Sawada N (2014) The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol 44(9):777–782. doi:10.1093/jjco/hyu096
Sasaki S, Kobayashi M, Ishihara J, Tsugane S, JHPC (2003) Self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study: questionnaire structure, computation algorithms, and area-based mean intake. J Epidemiol 13:S13–S22
Tsugane S, Kobayashi M, Sasaki S (2003) Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: comparison with dietary records for main nutrients. J Epidemiol 13:S51–S56
The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology (2005) Standard tables of food composition in Japan. 5th revised and enlarged edn. National Printing Bureau, Tokyo
Willett W (1998) Nutritional epidemiology. 2nd edn. Oxford University Press, New York
Sasaki S, Matsumura Y, Ishihara J, Tsugane S (2003) Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC study Cohort I to assess dietary fiber intake: comparison with dietary records. J Epidemiol 13 (1 Sup):S106–S114. doi:10.2188/jea.13.1sup_106
Sawada N, Iwasaki M, Yamaji T, Shimazu T, Sasazuki S, Inoue M et al (2015) Fiber intake and risk of subsequent prostate cancer in Japanese men. Am J Clin Nutr 101(1):118–125. doi:10.3945/ajcn.114.089581
Kokubo Y, Iso H, Saito I, Yamagishi K, Ishihara J, Inoue M et al (2011) Dietary fiber intake and risk of cardiovascular disease in the Japanese population: the Japan Public Health Center-based study cohort. Eur J Clin Nutr 65(11):1233–1241. doi:10.1038/ejcn.2011.100
Ministry of Education, Culture, Sports, Science and Technology (2000) International classification of diseases for oncology. 3rd edn. WHO, Geneva
Holmes MD, Liu S, Hankison SE, Colditz GA, Hunter DJ, Willett WC (2004) Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol 159(8):732–739. doi:10.1093/aje/kwh112
Liu Y, Colditz GA, Cotterchio M, Boucher BA, Kreiger N (2014) Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res Treat 145(2):461–470. doi:10.1007/s10549-014-2953-3
Gunter MJ, Xie X, Xue X, Kabat GC, Rohan TE, Wassertheil-Smoller S et al (2015) Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res 75(2):270–274. doi:10.1158/0008-5472.can-14-2317
Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Adlercreutz H, Wolk A (2008) Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status–a prospective cohort study among Swedish women. Int J Cancer 122(2):403–412. doi:10.1002/ijc.23060
Park Y, Brinton LA, Subar AF, Hollenbeck A, Schatzkin A (2009) Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 90:664–671. doi:10.3945/ajcn.2009.27758
World Health Organization (2014) Systematic review on diet, nutrition, physical activity and survival and second cancers in breast cancer survivors. Imperial College London, London
World Cancer Research Fund International Continuous Update Project Report (2010) Breast cancer 2010 report: Food, nutrition, physical activity, and the prevention of breast cancer. Imperial College London, London
Dahm CC, Keogh RH, Spencer EA, Greenwood DC, Key TJ, Fentiman IS et al (2010) Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 102(9):614–626. doi:10.1093/jnci/djq092
World Cancer Research Fund/American Institute for Cancer Research (2014) Guideline for breast cancer screening based on effectiveness evaluation 2013. National Cancer Center, Tokyo
Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T et al (2010) Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Res 12(5):R88. doi:10.1186/bcr2756
National Cancer Center (2012) Cancer fact sheets: breast cancer. https://gco.iarc.fr/today/fact-sheets-cancers?cancer=15&type=0&sex=2
International Agency for Research on Cancer (2015) Cancer incidence 3 prefectures (1985–2010). http://ganjoho.jp/reg_stat/statistics/dl/index.html
Acknowledgments
We would like to thank the Iwate, Ibaraki, Niigata, Kochi, Nagasaki, and Okinawa Cancer Registries for providing their incidence data.
Funding
This work was funded by the National Cancer Center Research and Development Fund [23-A-31(toku), 26-A-2] and Kiban A: Burden of Disease Fund [25253051].
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
M. Inoue is the beneficiary of a financial contribution from the AXA Research Fund as a chair holder of the AXA Department of Health and Human Security, Graduate School of Medicine, The University of Tokyo. The AXA Research Fund had no role in the design, data collection, analysis, interpretation or manuscript drafting, or in the decision to submit the manuscript for publication. All authors declare that they have no conflict of interest.
Additional information
JPHC Study Group members are listed at the following site: http://epi.ncc.go.jp/en/jphc/781/3838.html.
Rights and permissions
About this article
Cite this article
Narita, S., Inoue, M., Saito, E. et al. Dietary fiber intake and risk of breast cancer defined by estrogen and progesterone receptor status: the Japan Public Health Center-based Prospective Study. Cancer Causes Control 28, 569–578 (2017). https://doi.org/10.1007/s10552-017-0881-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-017-0881-3