Abstract
Aim
Physical activity after prostate cancer diagnosis has been shown to reduce the risk of disease progression. Here, we aimed to evaluate the effect of a 2-year home-based endurance training intervention on body composition, biomarkers levels, and prostate-specific antigen (PSA) doubling time as a surrogate end-point for progressing disease.
Methods
Out-clinic patients with either biochemical recurrence following radical prostatectomy or patients managed on active surveillance were randomized to either 24 months (3 times/week) of home-based endurance training or usual care. Aerobic fitness, body composition, insulin sensitivity, and biomarkers were measured at 0, 6, and 24 months of intervention. PSA doubling time (PSADT) was calculated based on monthly PSA measurements.
Results
Twenty-five patients were enrolled, and 19 patients completed the study. PSADT increased in the training group from 28 to 76 months (p < 0.05) during the first 6 months and was correlated with changes in VO2max (p < 0.01, r 2 = 0.41). The training group lost 3.6 ± 1.0 kg (p < 0.05) exclusively as fat mass, yet the changes in body composition were not associated with the increased PSADT. The training group showed significant improvements in plasma triglycerides, adiponectin, IGF-1, IGFBP-1, and fasting glucose levels, but no changes in insulin sensitivity (measured as Matsuda index), testosterone, cholesterols, fasting insulin, plasma TNF-alpha, IL-6, or leptin levels. The control group showed no changes in any of the evaluated parameters across the 2-year intervention.
Conclusion
In this small randomized controlled trial, we found that improvements in fitness levels correlated with increasing PSADT, suggesting a link between training and disease progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In epidemiological studies, physical activity following prostate cancer (PC) diagnosis has been shown to reduce the risk of PC progression and mortality [1, 2]. In these studies, Kenfield et al. [2] found a 61 % reduction in prostate cancer-specific death in subjects, who were engaged in vigorous activity more than 3 h/week compared with those engaged in less than 1 h/week after diagnosis. Similar effects were found in another trial, where brisk walking for more than 3 h/week following diagnosis decreased the risk of prostate cancer progression by 57 % [1].
These positive effects of physical activity have prompted large interest in engaging PC patients in exercise intervention studies. Yet, the vast majority of these exercise intervention studies have focused on patients managed with hormonal therapy, as these patients are exposed to widespread metabolic adverse effects, including increased body weight and fat mass, loss of lean body mass (LBM), and insulin resistance [3–6]. Exercise training in PC patients on androgen deprivation therapy (ADT) leads to increased fitness levels, reductions in body weight through decreases in fat mass, and maintenance of muscle mass and functional capacity [3–6]. Moreover, we have shown that 12 weeks of endurance training in PC patients receiving ADT could improve insulin resistance through increases in peripheral insulin sensitivity as evaluated by an euglycemic-hyperinsulinemic clamp with stable isotopes [7].
Based on the epidemiological data showing that physical activity increases the time to disease progression in PC patients, we aimed to randomize PC patients with recurrent PSA after radical prostatectomy or managed on active surveillance to a 2-year endurance training intervention or usual care, aiming to improve their fitness, body composition, biomarkers levels and increase PSA doubling time (PSADT).
Methods
Participants
Out-clinic patients with biochemical recurrence (PSA > 0.5 ng/ml) following radical prostatectomy or patients managed on active surveillance regardless of PSA level or biochemical progression were recruited from the Department of Urology at Rigshospitalet, Copenhagen, Denmark. The subjects underwent a medical examination and a standard blood test after 12 h of fasting to determine eligibility. Inclusion criteria: men ≥ 18 years of age with either (1) an elevation in PSA above 0.5 μg/l following radical prostatectomy for localized prostate cancer, or (2) undergoing active surveillance for prostate cancer with a Gleason score ≤ 6.3, tumor ≤ 2 biopsies, stage ≤ T2a and PSA ≤ 10. Patients were enrolled regardless of their prior physical activity levels, and prior physical activity levels were thus not formally registered. Exclusion criteria: (1) infections during the last 2 weeks; (2) severe cardiovascular diseases; (3) severe arthritis; (4) severe neuropathy; (5) severe hypertension; and (6) treatment other than radical prostatectomy or active surveillance. The patients were offered endocrine treatment if one of the following criteria was established: (1) confirmed metastatic disease and (2) PSADT < 9 months calculated by at least four measurements with more than 3 weeks in between. Written informed consent was obtained from all subjects according to the requirements from the local ethical committee (H-D-2008-015). The study was performed in accordance with the Helsinki Declaration.
Study protocol
Baseline testing consisted of a dual-energy X-ray absorptiometry (DXA) scan to determine body composition, and an incremental workload exercise test to determine fitness (VO2max) on day one, followed by an oral glucose tolerance test (OGTT) 48–96 h after on day two. After the two baseline visits, patients were randomized at a ratio of 1:2 to either control or training through a simple adaptive randomization procedure. Randomization was blinded to the referring physician and laboratory technicians, but not to the assessors of fitness test, OGTT, and DXA scanning. PSA measurements were repeated monthly during the intervention. PSA measurements performed before enrollment in the present study were used to calculate pre-intervention PSADT. Pre-intervention PSA measurements were found by review of medical records, and the latest 3–4 measurements before enrollment were used. For subjects, who had undergone prostatectomy, only post-operation PSA measurements were used. Evaluation of the intervention by the same test procedures was performed after 6 and 24 months. Again the OGTT was performed 48–96 h after the VO2max test to ensure that the measurements of insulin sensitivity were equally influenced by the last bout of exercise.
Training protocol
The subjects in the training group performed unsupervised endurance training 3 times per week for 45 min for 24 months at a heart rate corresponding to the desired percentage of maximal oxygen consumption (VO2max) measured during the incremental workload exercise test. The incremental workload exercise test was, in addition to baseline, measured in the training group after 3, 9, 12, and 18 months of training with the purpose of providing feedback and to adjust the absolute training work load. Furthermore, every month the patients came in for PSA determination, and at that point training adherence and progression were informally discussed. The training consisted of 5 min of warm-up, followed by 35 min of different interval training programs and 5 min of cool down. The intensity of the 35 min of interval training varied between 50 and 100 % of VO2max. The first week day of training, the training consisted of intervals of 5- to 10-min duration (intensity 55–75 % of VO2max), the second day of intervals of 5- to 25-min duration (intensity 55–75 % of VO2max), and the third day of intervals of 1- to 5-min duration (intensity 50–100 % of VO2max). The mean intensity was targeted at 60 % of VO2max from month 0 to 6 and 65 % of VO2max from month 7 to 24. The subjects were instructed to use a heart rate monitor on the day of group allocation by the training responsible researcher. Heart rate was continuously self-monitored during all training sessions (RS400, Polar, Kempele, Finland). Moreover, at each monthly control visit, the stored heart rate data were collected and analyzed by the training responsible researcher, and feedback on training intensity was given to the patients. The mode of training was self-elected and consisted of walking, running, rowing, swimming, and bicycling.
Control protocol
The subjects in the control group were encouraged not to make any changes in their everyday living. If they were performing any kind of training before participating in the present study, they were still allowed to do so during the study.
Training log
Training adherence was determined as the percentage of performed exercise session relative to the prescribed sessions. Subjects in both groups kept a paper-based training log, in which they daily recorded the number of steps taken. For this purpose, all subjects were handed out a pedometer (SW-701, Step Into Health, USA). Subjects in the training group also took notes about all training sessions, and all subjects registered any additional training. No data on food intake or diet were recorded.
Maximal oxygen consumption
The maximal oxygen consumption (VO2max) was determined via an incremental exercise test to volitional fatigue on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). VO2 was measured with an indirect calorimetric system (Quark b2; CosMed, Rome, Italy), recording data every 15 s. At least two of the following three criteria were necessary to establish that a true VO2max had been reached: (1) a respiratory exchange ratio ≥1.10, (2) a plateau in VO2 despite an increased workload, and (3) rpm < 60 in more than 10 s.
Body composition
Subjects were weighed wearing a T-shirt, boxers, and no shoes. Body weight was measured to the nearest 0.1 kg on a digital platform scale. Height was measured to the nearest 0.5 cm using a wall-mounted stadiometer. DXA scans (Lunar Prodigy, GE Medical Systems Wisconsin, USA, version 8.8) were performed to assess body composition regarding whole-body, trunk, android fat mass, and gynoid fat mass, as well as whole-body lean body mass.
Glucose tolerance and insulin sensitivity
A 2-h 75 g oral glucose tolerance test (OGTT) was performed following an overnight fast. Blood samples were obtained at 0, 30, 60, 90, and 120 min for the measurements of plasma glucose and plasma insulin. Area under the glucose and insulin response curves was calculated by the trapezoid rule. Insulin sensitivity during OGTT was estimated by the Matsuda index [8]. The homeostatic model assessment of insulin resistance (HOMA-IR) was also calculated [9]. The subjects were not allowed to perform any form of physical activity the day before the OGTT.
Laboratory analysis
Plasma lipids (total cholesterol, LDL-C, HDL-C, and triglyceride) were measured at baseline and after 6 and 24 months of training. Plasma concentrations of tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, adiponectin, leptin, insulin growth factor (IGF)-I, and insulin growth factor-binding protein (IGFBP)-I were measured at baseline and after 24 months. TNF-α, IL-6, adiponectin, and leptin were determined by ELISA (Meso Scale Discovery, Gaithersburg, MD, USA). Plasma concentrations of IGF-I and IGFBP-I were determined by ELISA (R&D systems, Minneapolis, MN, USA). All determinations were run as duplicates, and mean values were calculated. PSA, testosterone, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, insulin, and glucose were determined using standard laboratory procedures (Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark).
Calculations
PSADT in months was calculated as the natural log of 2 divided by the PSA slope (www.nomogram.org).
Statistical analysis
Data were analyzed using SAS 9.1.3 (SAS Institute Inc. USA). Data are presented as mean ± SEM in the figures. All outcome variables were checked for normal distribution by histogram plots and by Kolmogorov–Smirnov analysis and were log-transformed to achieve an approximate normal distribution when necessary. Unpaired t tests were performed to assess between-group differences at baseline. Changes within groups (from month 0 to 6 and 0 to 24) and between groups were analyzed using repeated measures (two-way ANOVA). If a significant interaction (group × time) was found, Dunnett-corrected post hoc tests were applied to identify significant differences within group and between groups. Paired t tests were performed for PSADT to identify significant differences over time within each group. p < 0.05 was considered statistical significant.
Results
Baseline characteristics
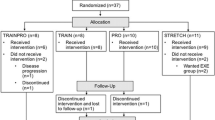
Of the 28 recruited subjects, two declined to participate and one did not fulfill the inclusion criteria (Fig. 1). Accordingly, 25 subjects were randomized to either the training or the control group. Four subjects, three in the training group and one in the control group, started endocrine treatment within 6 months of intervention and were excluded from the study. Furthermore, two subjects from the training group were unable to perform training due to sickness unrelated to prostate cancer, and they were also excluded after 6 months of intervention. Five of the excluded patients belonged to the group of patients with biochemical recurrence and one was on active surveillance. Twelve patients in the training group and seven patients in the control group completed the 24 months of intervention. Five of the excluded patients took part in the first 6 months of the intervention, and they did not differ in any of the evaluated parameters compared with the completing patients.
Baseline characteristics of the training and control groups are given in Table 1. At baseline, no differences in VO2max, body composition, lipid profile, inflammation status, or insulin sensitivity were found. The PSA concentration and PSADT were, furthermore, equal between the two groups at baseline. One subject in the training group and four subjects in the control group had impaired glucose tolerance, and one was diabetic in the control group, according to the criteria of the World Health Organization and International Diabetes Federation.
Training and daily stepping
Adherence to the training program based on performed training session was 88.2 ± 17.4 % from month 0 to 6 and 89.9 ± 6.2 % from month 7 to 24 in the training group (Fig. 2a), and the mean training intensity was 61 ± 8 % of VO2max from month 0 to 6 and 64 ± 12 % of VO2max from month 7 to 24 (Fig. 2b). The mean number of steps per day, including steps during the training sessions, was 8,961 ± 2,904 and 8,776 ± 3,280 in the training group from month 0 to 6 and month 7 to 24, respectively (Fig. 2c). The control group took a mean number of steps per day of 7,477 ± 3,222 and 6,120 ± 1,889 from month 0 to 6 and month 7 to 24, respectively (Fig. 2c).
Training adherence and fitness levels. For the training group, a training adherence to described exercise and b training intensity in training sessions in 0–6 and 7–24 months, respectively. c Mean daily steps per day in the control and training groups. d Changes in VO2max expressed as ml(O2)/min/kg and e VO2max expressed as ml(O2)/min after 0–6 and 0–24 months of intervention in the control and training groups. Data are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA with post hoc t test. *p < 0.05 for an effect of the intervention between the groups or changes within the training group
After 6 months of intervention, the training group showed a significant increase in fitness levels (p < 0.05, Fig. 2d) and a tendency to an increase was observed in the same group after 24 months (p = 0.09, Fig. 2d). The change (0–6) in fitness levels was significantly different between the two groups (p < 0.01). When body composition was not taken into consideration, there was a borderline difference between the changes in VO2max (ml(O2)/min) for the training and control groups (p = 0.08, Fig. 2e, Table 2).
Body composition
Patients in the training group lost 2.5 ± 0.9 and 3.6 ± 1.0 kg body weight after 6 and 24 months of training (Fig. 3a), respectively, with no significant changes in lean body mass (Fig. 3b). Moreover, in the training group, total fat mass decreased with 3.1 ± 3.0 kg (p < 0.01, Fig. 3c) and 4.4 ± 3.8 kg (p < 0.001); trunk fat mass decreased with 2.0 ± 1.7 kg (p < 0.01, Fig. 3d) and 2.4 ± 2.3 kg (p < 0.001); gynoid fat mass decreased with 0.5 ± 0.5 kg (p < 0.01, Fig. 3e) and 0.6 ± 0.6 kg (p < 0.001); and android fat mass decreased with 0.3 ± 0.4 kg (p < 0.05, Fig. 3f) and 0.5 ± 0.5 kg (p < 0.01) after 6 and 24 months of training, respectively. ∆0–6 and ∆0–24 in fat mass, trunk fat mass, gynoid fat mass, and android fat mass were significantly different between the two groups (all, p < 0.05).
Changes in body composition. Changes in body weight (a), lean body mass (b), total fat mass (c), trunk fat mass (d), gynoid fat mass (e), and android fat mass (f) after 0–6 and 0–24 months of intervention in the control and training groups. Data are expressed as mean ± SEM. Statistical significance was determined by two-way ANOVA with post hoc t test. *p < 0.05 for an effect of the intervention between the groups, or *p < 0.05, **p < 0.01 or ***p < 0.001 for changes within the training group
PSA doubling time
Within the training group, PSADT increased significantly from pre-training to after 6 months of training (p = 0.04, Table 2), while there was no change in PSADT within the control group (Table 2). There was a borderline difference between the changes in PSADT in the training and control groups at 6 months (p = 0.06). For correlation analyses, we broke the randomization and pooled data from the control and training groups due to the low number of subjects. The change in PSADT within the first 6 months correlated with the change in fitness levels (R 2 = 0.29, p = 0.0185, Fig. 4a) and change in VO2max (R 2 = 0.41, p < 0.003, Fig. 4b). No correlations were seen between change in PSADT and change in fat mass or LBM (Fig. 4c, d). There was no significant change in PSA levels at 6 or 24 months in either group (Table 2).
PSADT and correlations to fitness and body composition. Prostate-specific antigen doubling time (PSADT) was correlated to changes in a fitness levels, b VO2max, c total fat mass, and d lean body mass. Data were pooled for both the training group (closed circles) and control group (open circles) (n = 19)
Biomarkers and insulin sensitivity
After 24 months of training, the training group displayed significant reductions in plasma triglycerides (p < 0.01) and IGF-1 (p < 0.05) levels and increases in plasma adiponectin (p < 0.05) and IGFBP-I (p < 0.05, Table 2). No changes were found for testosterone, total cholesterol, LDL, HDL, TNF-alpha, IL-6, or leptin in the training group. In the control group, none of the evaluated biomarkers changed over time.
Fasting plasma glucose levels decreased with 24 months of training (p < 0.05), while no change was seen in the control group. The change in area under the curve for insulin after 24 months tended to be different between the two groups (p = 0.06) (Table 2); otherwise, no changes in fasting plasma insulin levels, the area under the curve for plasma glucose, or the indices for insulin sensitivity (HOMA-IR and Matsuda) were observed in neither of the groups.
Discussion
The present small randomized controlled trial aimed to investigate the possible effects on PSA doubling time (PSADT) and metabolic parameters of home-based endurance training in out-clinic prostate cancer patients with biochemical recurrence following radical prostatectomy or patients managed on active surveillance. Six months of endurance training significantly increased PSADT and a borderline significant increase in PSADT when the training group was compared to the control group. These data provide support for the epidemiological finding that physical activity may delay cancer progression. As expected, endurance training had beneficial effects on fitness and body composition. Moreover, PSADT strongly correlated with changes in VO2max but not with changing in body composition, suggesting that the protective effect of exercise on PC progression is linked with improved fitness levels rather than with changes in body composition.
Lifestyle intervention studies have previously reported a decrease in PSA concentration and cancer-related clinical events in 93 prostate cancer patients managed on active surveillance after 12–24 months of combined increased physical activity and dietary modification [10, 11]. In contrast, Hébert et al. [12] found no change in PSA concentration after a similar lifestyle intervention for 6 months in 47 patients with recurrent disease following curatively intended therapy. Even though the value of repeated PSA measurements is controversial, PSADT is still applicable as a surrogate marker for disease progression and is routinely used to monitor patients managed on active surveillance as well as to monitor patients prior to ADT [13–16]. In contrast to the lifestyle intervention studies, we evaluated the isolated effect of exercise training, and the increase in PSADT in PC patients after endurance training may suggest that the training component is crucial for the effect on PSA levels.
In concordance with previous studies on training and lifestyle intervention in patients with biochemically recurrence following radical prostatectomy or on active surveillance [19–21] and endurance training in prostate cancer patients managed with ADT [22], we found that endurance training increased fitness levels, lowered body weight through reductions in fat mass, and maintained muscle mass. Diminished cardiorespiratory fitness is linked with impaired functional capacity in cancer patients [23], providing support for promoting physical rehabilitation in cancer patients in order to reduce tumor and therapy-related symptoms. Here, we showed that improvements in fitness levels were correlated with increments in PSADT. In line with this finding, recent reports have showed that physical inactivity levels are associated with an increase in PSA [24, 25]. Even though these associations do not give any mechanistic insight into the protective effects of exercise, it suggests that the intrinsic systemic changes induced during physical exertion, which give raise to improvements in VO2max, are also involved in the molecular suppression of tumor progression. Of note, cell culture incubation studies with exercise-conditioned serum have shown that the systemic changes occurring during a bout of exercise can directly inhibit prostate cancer growth [26].
Muscle dysfunction, characterized by loss of muscle mass and function, has been associated with critical clinical end-points across numerous cancer diagnoses [27]. In this study, neither the training nor the control group had any changes in LBM, and thus, we found no correlations between LBM and PSADT in this study. Obesity is associated with higher biochemical recurrence rates following radical prostatectomy and increased risk of prostate cancer-specific death [28, 29]. A weight gain of more than 2.2 kg following radical prostatectomy increases the risk of cancer recurrence [30], suggesting that changes in obesity after diagnosis possess a modifying effect on the progression of established prostate cancer. We showed reductions in whole-body fat mass of 3.1 and 4.4 kg after 6 and 24 months of training, respectively, and even though these reductions in fat mass were not associated with increases in PSADT, they certainly have beneficial effects on the patients’ overall metabolic health.
Training-induced reductions in systemic inflammatory cytokines, sex hormones, adipokines, and the insulin signaling axis have been suggested as molecular mechanisms whereby exercise could exert its protective effect on prostate cancer progression [17]. In this study, the intervention did not change the concentrations of the pro-inflammatory cytokines, TNF-α and IL-6; the sex hormone testosterone or the adipokine, leptin, indicating that exercise effects on resting levels of these parameters did not mediate the beneficial effect on cancer progression in the present study. We did, however, observe an effect across time on IGFBP-I and IGF-I. Circulating IGFBP-I binds IGF-I with high affinity, resulting in less IGF-I to interact with the IGF-I receptor (IGF-IR) [18]. The suggested cancer-promoting effects of IGF-I is depending on the downstream signaling of IGF-I binding to the IGF-IR on the cell membrane. The increased levels of IGFBP-I and decreased levels of IGF-I may therefore have impaired signaling after 24 months of training.
There are several limitations of the present study. First of all, the small number of subjects results in low statistical power, which might conceal some of the possible effects of the intervention. The low number of subjects was reflected in very large variations in PSADT, which make it further difficult to identify an effect of the intervention in the controlled design. Secondly, the control group maintained habitual activity habits, and this group does therefore not represent an entirely inactive contrast to the training group. In fact, the mean number of steps per day was similar between the two groups, which may suggest that the difference in total physical activity was too little to allow for larger differences to be detected in PSADT. Thirdly, training status of the patients before enrollment in the study was not controlled for, and some of the patients led a very active lifestyle before commencing in the present study, as also reflected by high VO2max values at baseline.
The study had a very high adherence to the training, resulting in marked improvements in physiological parameters. Our experience was that this group of patients had very few symptoms from their disease, but were living with a constant fear of disease progression. Thus, they wholly embraced the prescribed training as a means to do something active about their situation. In contrast to PC patients in ADT, much fewer exercise intervention studies have been performed in this group of PC patients with biochemical recurrence or on active surveillance. Our results suggest that exercise training in this group is both well accepted with high training adherence and may have effects on disease progression, as measured by PSADT. Yet, both parts need to be validated in larger RCT studies.
In conclusion, the present pilot study demonstrated that a home-based endurance training programme is a feasible option for improving fitness, body composition, and metabolism in this particular group of prostate cancer patients. Of particular interest, the present study demonstrated a promising tendency toward improvement in PSADT, adding some support to the idea that increased level of physical activity leading to improved fitness level may be a factor contributing to delay prostate cancer progression.
References
Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM (2011) Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res 71(11):3889–3895
Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM (2011) Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 29(6):726–732
Payton S (2013) Prostate cancer: optimizing exercise interventions for men on ADT. Nat Rev Urol 10(11):615
Culos-Reed SN, Robinson JW, Lau H et al (2010) Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer 18(5):591–599
Segal RJ, Reid RD, Courneya KS et al (2003) Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 21(9):1653–1659
Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU (2010) Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 28(2):340–347
Hvid T, Winding K, Rinnov A et al (2013) Endurance training improves insulin sensitivity and body composition in prostate cancer patients treated with androgen deprivation therapy. Endocr Relat Cancer 20(5):621–632
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22(9):1462–1470
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Ornish D, Weidner G, Fair WR et al (2005) Intensive lifestyle changes may affect the progression of prostate cancer. J Urol 174(3):1065–1069
Frattaroli J, Weidner G, Dnistrian AM et al (2008) Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology 72(6):1319–1323
Hebert JR, Hurley TG, Harmon BE, Heiney S, Hebert CJ, Steck SE (2012) A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol 36(2):e128–e136
Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H (2001) PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc 76(6):576–581
Klotz L (2006) Active surveillance with selective delayed intervention for favorable risk prostate cancer. Urol Oncol 24(1):46–50
Thomsen FB, Brasso K, Klotz L, Røder MA, Berg KD, Iversen P (2014) Active surveillance for clinically localized prostate cancer—a systematic review. J Surg Oncol 109(8):830–835
Thomsen FB, Christensen IJ, Brasso K, Røder MA, Iversen P (2014) Prostate-specific antigen doubling time as a progression criterion in an active surveillance programme for patients with localized prostate cancer. BJU Int 113(5b):E98–E105
McTiernan A (2008) Mechanisms linking physical activity with cancer. Nat Rev Cancer 8(3):205–211
Baxter RC (2014) IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 14(5):329–341
Ornish D, Lin J, Chan JM et al (2013) Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 14(11):1112–1120
Park S, Kim TN, Nam J et al (2012) Recovery of overall exercise ability, quality of life, and continence after 12 week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomised controlled study. Urology 80:299–305
Zopf EM, Bloch W, Machtens S, et al (2015) Effects of a 15-months supervised exercise program on physical and psychological outcomes in prostate cancer patients following prostatectomy: the ProReHab study. Intergr Cancer Ther 14(5):409–418
Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 32(4):335–346
Lakoski SG, Eves ND, Douglas PS, Jones LW (2012) Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 9(5):288–296
Loprinzi PD, Kohli M (2013) Effect of physical activity and sedentary behavior on serum prostate-specific antigen concentrations: results from the National Health and Nutrition Examination Survey (NHANES), 2003–2006. Mayo Clin Proc 88(1):11–21
Burton AJ, Martin RM, Donovan JL et al (2012) Associations of lifestyle factors and anthropometric measures with repeat PSA levels during active surveillance/monitoring. Cancer Epidemiol Biomark Prev 21(10):1877–1885
Rundqvist H, Augsten M, Strømberg A et al (2013) Effect of acute exercise on prostate cancer cell growth. PLoS ONE 8(7):e67579
Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P (2014) Muscle dysfunction in cancer patients. Ann Oncol 25(5):947–958
Cao Y, Ma J (2011) Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res 4(4):486–501
Freedland SJ, Platz EA (2007) Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev 29:88–97
Joshu CE, Mondul AM, Menke A et al (2011) Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res 4(4):544–551
Acknowledgments
Thomas S. Grøndahl and Lisa Gruschy are acknowledged for their help with various aspects of the present study. Ruth Rousing is acknowledged for technical assistance. During the study period, the Centre of Inflammation and Metabolism (CIM) was supported by a grant from the Danish National Research Foundation (DNRF55). The Centre for Physical Activity Research (CFAS) is supported by a grant from Trygfonden. KW was supported by the Danish Diabetes Academy. This study was further supported by the Danish Medical Research Council, and by grants from TrygFonden. The Copenhagen Muscle Research Centre (CMRC) is supported by a grant from the Capital Region of Denmark.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hvid, T., Lindegaard, B., Winding, K. et al. Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control 27, 165–174 (2016). https://doi.org/10.1007/s10552-015-0694-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0694-1