Abstract
Purpose
Diet after prostate cancer diagnosis may impact disease progression. We hypothesized that consuming saturated fat after prostate cancer diagnosis would increase risk of mortality, and consuming vegetable fat after diagnosis would lower the risk of mortality.
Methods
This was a prospective study among 926 men with non-metastatic prostate cancer in the Physicians’ Health Study who completed a food frequency questionnaire a median of 5 years after diagnosis and were followed for a median of 10 years after the questionnaire. We examined post-diagnostic saturated, monounsaturated, polyunsaturated, and trans fat, as well as animal and vegetable fat, intake in relation to all-cause and prostate cancer-specific mortality. Hazard ratios (HR) and 95 % confidence intervals (CI) were estimated using multivariate Cox proportional hazards regression.
Results
We observed 333 deaths (56 prostate cancer deaths) during follow-up. Men who obtained 5 % more of their daily calories from saturated fat and 5 % less of their daily calories from carbohydrate after diagnosis had a 1.8-fold increased risk of all-cause mortality (HR 1.81; 95 % CI 1.20, 2.74; p value 0.005) and a 2.8-fold increased risk of prostate cancer-specific mortality (HR 2.78; 95 % CI 1.01, 7.64; p value 0.05). Men who obtained 10 % more of their daily calories from vegetable fats and 10 % less of their daily calories from carbohydrates had a 33 % lower risk of all-cause mortality (HR 0.67; 95 % CI 0.47, 0.96; p value 0.03).
Conclusions
Among men with non-metastatic prostate cancer, saturated fat intake may increase risk of death and vegetable fat intake may lower risk of death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second leading cause of cancer death among men, and over 2.5 million men currently live with prostate cancer in the USA [1]. Growing, but still limited, evidence suggests that lifestyle factors after diagnosis, including smoking [2], physical activity [3], body weight [4], and diet [5], may affect risk of disease progression and survival among men with prostate cancer. We recently reported that men who obtained 10 % more of their daily calories from vegetable fat and 10 % less of their daily calories from carbohydrate after diagnosis of non-metastatic prostate cancer had a 29 % lower risk of lethal prostate cancer and a 26 % lower risk of death from all causes [5]. Saturated fat intake assessed at diagnosis has also been associated with a higher risk of biochemical recurrence and prostate cancer-specific mortality [6–8].
To further evaluate the potential role of dietary fat in the progression of prostate cancer, we prospectively examined post-diagnostic intake of saturated, monounsaturated, polyunsaturated, trans fat, as well as animal and vegetable fat, in relation to all-cause mortality among 926 men diagnosed with non-metastatic prostate cancer in the Physicians’ Health Study. Prostate cancer-specific death was considered a secondary outcome due to the small number of events (n = 56). We hypothesized that vegetable fat intake after diagnosis would be associated with lower risk of all-cause and prostate cancer-specific mortalities, while saturated fat intake would be associated with increased risk of these outcomes.
Materials and methods
Study population
The Physicians’ Health Study (PHS) was a randomized trial of aspirin and beta-carotene initiated in 1982 among 22,071 male US physicians. The aspirin intervention was stopped early in 1988 due to the benefits of aspirin on myocardial infarction; the beta-carotene intervention was stopped as planned in 1995. In 1997, the Physicians’ Health Study II (PHS-II) was initiated among 14,641 male US physicians, 7,641 of whom had participated in PHS. PHS-II randomized men to vitamin C, vitamin E, beta-carotene, or multivitamin until 2011. In total, 29,071 male US physicians participated in PHS, PHS-II, or both and are actively followed for disease endpoints. This study was approved by the Institutional Review Boards of Partners HealthCare and the Harvard School of Public Health.
Identification of prostate cancer cases
Men were asked every year whether they had been diagnosed with prostate cancer [9]. If a man reported a prostate cancer diagnosis, we sought medical records to verify the diagnosis and recorded stage and grade, prostate-specific antigen (PSA) levels, and treatments.
Outcome assessment and follow-up
The main outcome for this analysis was death from all causes; prostate cancer-specific mortality was examined as a secondary endpoint due to the small number of events (n = 56). Deaths were ascertained via mail, telephone, and review of the National Death Index; mortality follow-up is 99 % complete [10]. The PHS Endpoints Committee of study physicians confirmed cause of death via medical records and death certificates. A man was considered to have died of prostate cancer if prostate cancer metastases were present and no more plausible cause of death was mentioned. Medical records and/or death certificates were not available for six of the men reported as having died due to prostate cancer (11 %). These deaths were categorized as un-refuted by the endpoints committee upon review of all other available data and retained as events in the main analysis; our results were unchanged in sensitivity analyses excluding these six men.
Dietary assessment
Post-diagnostic fat intake was assessed using a validated food frequency questionnaire (FFQ) administered between 1997 and 2001 [11]. Men were asked to report their usual intake of 61 foods and beverages over the previous year in nine frequency categories ranging from never or <1/month to 6+/day. A common portion size was specified for each food item (e.g., 1 oz of nuts). Nutrient data came from the US Department of Agriculture. Intakes of each of the fats of interest were calculated by multiplying the amount of each type of fat in the specified portion size of a food item by the frequency of intake of that food item and summing across all foods. To calculate animal fat, total fat in the specified portion size of each food item from animal sources was multiplied by the frequency of intake for each item and summed across all food items. To calculate vegetable fat, total fat in the specified portion size of food items from vegetable sources was multiplied by the frequency of intake of each item and summed across all food items. For food items with both animal and vegetable components (e.g., mashed potatoes, pizza, etc.), the fat content from animal and vegetable sources was determined based on a standard recipe. We multiplied intake of each of the fats (g/d) by 9 kcal and divided by total calories per day to calculate the percent of daily calories from each of the fats of interest.
The FFQ was validated among a similar cohort of 127 men from the Health Professionals Follow-up Study [12]. Men completed two FFQs 1 year apart and two 7-day diet records within the same year. The de-attenuated correlation coefficients between the FFQ and diet records were: 0.75 for saturated fat, 0.37 for polyunsaturated fat, and 0.68 for monounsaturated fat. A subset of men provided subcutaneous fat aspirates, and the correlation between the FFQ and fat aspirate concentrations was 0.50 for polyunsaturated fat and 0.29 for trans fat [13], respectively.
Inclusion criteria
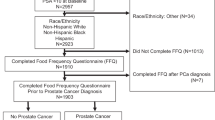
Men in this analysis were initially diagnosed with non-metastatic prostate cancer after enrollment in PHS or PHS-II and prior to completing the FFQ in 1997–2001 (n = 926).
Statistical analysis
We used Cox proportional hazards regression to prospectively examine post-diagnostic saturated, monounsaturated, polyunsaturated, and trans fat, as well as animal and vegetable fat, intake and risk of all-cause mortality. Person-time was calculated from date of the FFQ to death or end of follow-up (1 January 2009 or return of the last available questionnaire if after 1 January 2009), whichever came first. We used calendar time in days as our timescale and adjusted for years between diagnosis and completion of the FFQ.
We examined the impact of consuming higher amounts of fat and lower amounts of carbohydrate, the largest contributor to calories in the US diet, while holding total calorie intake constant using the multivariate nutrient density method [11, 14]. To do so, we included percent energy from protein, alcohol, and each of the fats in our multivariate model along with energy and other covariates (described below) and omitted carbohydrate. Thus, the effect estimate for the fat of interest can be interpreted as the effect of increasing calories from that fat and decreasing calories from carbohydrate by the same amount. In a secondary analysis, we examined the effect of higher vegetable fat and lower animal fat intake after prostate cancer diagnosis holding total calories constant. In this model, we included percent energy from protein, alcohol, carbohydrate, trans fat, and vegetable fat in addition to calories and other covariates. Thus, the effect estimate for vegetable fat can be interpreted as increasing calories from non-trans vegetable fat and decreasing calories from non-trans animal fat. We included trans fat in the model in order to examine the effect of vegetable fat excluding trans fat, due to the known adverse health effects of trans fat and its decreasing prevalence in the food supply. We examined the effect of fat intake in quartiles using indicator variables with the lowest quartile as the reference, and tested for evidence of a linear trend by modeling the median of the quartiles as a continuous term.
Our first model was adjusted for age at diagnosis (years), time from diagnosis to FFQ (years), and calories (kcal/d). Our multivariate model was additionally adjusted for modified D’Amico risk category (high: clinical T-stage T3 or higher and PSA at diagnosis >20 ng/ml or biopsy Gleason sum >7; else intermediate: clinical T-stage T1 or T2 and either 10 < PSA at diagnosis ≤20 or biopsy Gleason sum = 7; else low: clinical T-stage T1 or T2 and PSA at diagnosis ≤10 ng/ml and biopsy Gleason sum ≤7), primary treatment (radical prostatectomy, radiation therapy, hormonal therapy, other), body mass index (BMI; continuous), smoking (never, ever), and intake of alcohol (percent calories), protein (percent calories), and other fats (percent calories). We combined former and current smokers, because there were few current smokers in our population (n = 23). In addition, we conducted a sensitivity analysis excluding men who died within 2 years of the FFQ to examine whether reverse causation was affecting our results (i.e., men may change their diet prior to death as a result of underlying disease), and tested for evidence of effect modification by time between diagnosis and the FFQ using a Wald test of the cross-product term between time from diagnosis to the FFQ (dichotomized at the median of 5 years) and continuous fat intakes in our multivariate models.
SAS version 9.3 was used for all statistical analyses, and two-sided p values <0.05 were considered statistically significant.
Results
We observed 333 deaths among 926 men followed for a median of 10 years after the FFQ [interquartile range (IQR) = 8–11 years]. Cardiovascular disease was the leading cause of death (n = 70; 21 %); prostate cancer accounted for 56 deaths (17 %). The median time from diagnosis to the FFQ was 5 years (IQR = 2–8 years).
Men who consumed the most animal fat were older, had a higher BMI, were more likely to be current smokers and diagnosed with clinical stage T3, and less likely to be treated with radical prostatectomy than men who consumed the least animal fat (Table 1). Men with higher vegetable fat intake were less likely to be current smokers at diagnosis and have a Gleason sum 2–6, and more likely to be treated with radical prostatectomy than men who consumed the least vegetable fat.
Saturated fat intake after prostate cancer diagnosis was positively associated with risk of death (Table 2). Men in the highest quartile of saturated fat intake after diagnosis had a twofold higher risk of death from all causes compared with men in the lowest quartile [hazard ratio (HR) 2.08; 95 % confidence interval (CI) 1.16, 3.72; p-trend = 0.007]. In the continuous model, obtaining 5 % more calories from saturated fat and 5 % less calories from carbohydrate was associated with an 81 % increased risk of death (HR 1.81; 95 % CI 1.20, 2.74; p value 0.005). In contrast, men in the fourth quartile of vegetable fat intake after diagnosis had a 35 % lower risk of death compared with men in the first quartile (HR 0.65; 95 % CI 0.45, 0.93; p-trend = 0.03). Obtaining 10 % more calories from vegetable fat and 10 % less calories from carbohydrate after diagnosis was associated with a 33 % lower risk of death (HR 0.67; 95 % CI 0.47, 0.96; p value 0.03). We also examined the effect of consuming more vegetable fat and less animal fat (instead of less carbohydrate). Men who consumed 10 % more calories from vegetable fat and 10 % less calories from animal fat had a 44 % lower risk of death (HR 0.56; 95 % CI 0.38, 0.80; p value 0.002) (results not shown in Table). No other fats were significantly associated with risk of death. The results were unchanged in sensitivity analyses excluding the 35 men who died within 2 years of completing the FFQ (saturated fat—HR highest vs. lowest quartile: 2.17; 95 % CI 1.16, 4.06; p-trend = 0.01; vegetable fat—HR highest vs. lowest quartile: 0.66; 95 % CI 0.45, 0.97; p-trend = 0.04), and there was no evidence of effect modification by time between diagnosis and the FFQ.
We also observed an increased risk of prostate cancer-specific death among men who consumed greater amounts of saturated fat after diagnosis (Table 3). Men who obtained 5 % more calories from saturated fat and 5 % less calories from carbohydrate had a 2.8-fold higher risk of prostate cancer-specific mortality (HR 2.78; 95 % CI 1.01, 7.64; p value: 0.05). The results were not statistically significant in the categorical model, but there were only 56 prostate cancer-specific deaths observed during follow-up, limiting our power to examine this outcome.
Discussion
Men with higher levels of saturated fat intake after prostate cancer diagnosis had increased risk of death from all causes, and men with greater vegetable fat intake had decreased risk of death. These results replicate the benefits of vegetable fats for men with prostate cancer that we recently reported in the Health Professionals Follow-up Study [5]. Clinical trials evaluating the effects of vegetable fats and recommendations for men with prostate cancer to increase consumption of vegetable fats and decrease consumption of saturated fats may be warranted.
The prior report by our group is the only other study that has examined post-diagnostic fat intake in relation to overall survival among men with prostate cancer, and the results of this analysis are largely consistent with our previous findings [5]. In that study, men who replaced 10 % of calories from animal fat with vegetable fat after diagnosis had a 44 % lower risk of death from all causes (HR 0.66; 95 % CI 0.54, 0.81; p value < 0.001). The corresponding HR in the PHS population was 0.56 (95 % CI 0.38, 0.80; p value 0.002). Clinical trials are needed to determine the feasibility of this dietary change among men with prostate cancer; however, it is plausible that men could achieve and sustain this magnitude of dietary change over time. Assuming a man consumes 2,400 calories per day, replacing 10 % of daily calories from animal fat with vegetable fat could be achieved by using approximately two tablespoons of olive oil instead of two tablespoons of butter when cooking and at the table throughout the day. The strength of the association and its consistency in two independent populations provides strong support for further work investigating the feasibility and effect of interventions aimed at increasing vegetable fat intake in men with prostate cancer.
Fats from vegetable sources include a mix of fatty acids. Based on data from the 2010 administration of the Health Professionals Follow-up Study and the Nurses’ Health Study questionnaires, which utilize a similar FFQ, vegetable fat in our study population is approximately 42 % monounsaturated fat, 32 % polyunsaturated fat, 18 % saturated fat, and 8 % trans fat. When we examined these fatty acids in relation to all-cause and prostate cancer-specific mortalities, only saturated fat was significantly associated with either endpoint. Monounsaturated fat was suggestively inversely associated with all-cause and prostate cancer-specific mortality, but was not statistically significant (all-cause mortality HR 0.68; 95 % CI 0.42, 1.11; prostate cancer-specific mortality HR 0.52; 95 % CI 0.16, 1.63, respectively). N-6 polyunsaturated fats were suggestively inversely associated with all-cause mortality (HR 0.72; 95 % CI 0.36, 1.44), but not with prostate cancer-specific mortality (HR 1.01; 95 % CI 0.20, 5.09). While n-3 polyunsaturated fats were not associated with all-cause mortality (HR 0.99; 95 % CI 0.38, 2.59), they were suggestively associated with prostate cancer-specific mortality (HR 0.63; 95 % CI 0.06, 6.82), albeit with very wide confidence intervals. It is possible that the observed benefit of vegetable fat may be due to some other nutrient(s) or phytochemical(s) in food sources of vegetable fat. In a mouse model of prostate cancer, mice fed 100 g fat from whole walnuts or walnut oil had smaller prostate tumors compared with mice fed 100 g of fat blended to match the fatty acid profile of walnuts [15]. This supports the hypothesis that the benefit of vegetable fats may be driven by compound(s) in the fat component of food sources of vegetable fat, rather than the fatty acids themselves.
However, in contrast to monounsaturated and polyunsaturated fatty acids, we did observe a positive association between saturated fat intake after diagnosis and prostate cancer-specific death. Two out of three prior small studies examining fat intake in relation to prostate cancer-specific survival also reported that saturated fat intake was associated with higher risk of prostate cancer death [6, 8, 16]. Epstein et al. [6] reported that myristic acid and shorter-chain (C4–C10) saturated fatty acid intake were associated with more than twofold increases in risk of prostate cancer-specific mortality among Swedish men initially diagnosed with localized disease (myristic acid HR 2.39; 95 % CI 1.06, 5.38; shorter-chain saturated fatty acids HR 2.88; 95 % CI 1.24, 6.67). Meyer et al. [8] also reported that men in the highest tertile of saturated fat intake had a threefold increased risk of prostate cancer-specific mortality compared with men in the lowest tertile (95 % CI 1.3, 7.7; p value 0.008).
Together, our data support recommendations for men with prostate cancer to lower saturated fat (predominately from animal sources) and increase fat from vegetable sources. There are many plausible biologic mechanisms by which replacing fat from animal sources with fat from vegetable sources after prostate cancer diagnosis may lower risk of death, including modulation of inflammatory, oxidative stress, and energy metabolism pathways. Randomized controlled trials have reported that consuming vegetable fats in the form of nuts or olive oil increases circulating antioxidants and decreases markers of inflammation [17–26]. Furthermore, a Mediterranean diet supplemented with olive oil or mixed nuts reduced risk of incident cardiovascular disease [27], which was the most common cause of death in our study population of men with prostate cancer. Our results are in line with the known benefits of vegetable fats for cardiovascular disease risk and suggest that men diagnosed with prostate cancer should be counseled to follow a heart-healthy diet that replaces fats from animal sources with fats from vegetable sources.
Growing data suggest that food sources of vegetable fats many have a beneficial effect on prostate tumors as well. Men randomized to flaxseed supplementation prior to radical prostatectomy had lower proliferation rates in their tumors compared with men randomized to control [28]. Additionally, animal data suggest that components in olive oil induce apoptosis [29, 30] and inhibit migration, invasion, and adhesion of prostate cancer cells [31], and walnuts reduce prostate tumor growth [15, 32, 33] and inhibit androgen receptor expression in prostate cancer cells [34]. Oxidative stress and inflammatory pathways are hypothesized to have a role in prostate cancer progression [35, 36], and based on the data from cardiovascular disease, replacing fat from animal sources with vegetable fat modulates activities of these pathways systemically. Future research elucidating the biologic effects of reducing saturated fat and increasing fat from vegetable sources in men with prostate cancer would be of interest.
Limitations of this analysis include the small sample size with few events of prostate cancer-specific mortality, single assessment of post-diagnostic diet, lack of pre-diagnostic dietary data, and a homogeneous study population. However, our analysis of all-cause mortality remains highly informative for the potential development of clinical and public health recommendations for men with prostate cancer. Our single assessment of post-diagnostic diet may not be representative of our participants’ long-term post-diagnostic diet. Nevertheless, there is less variation over time in macronutrient intake relative to other nutrients, and the questionnaire likely adequately ranks participants’ post-diagnostic fat intake. Further, any error is likely non-differential with respect to the outcome due to our prospective assessment. In addition, we were unable to adjust for pre-diagnostic dietary behaviors and thus cannot conclude that the associations we observed were independent of fat intake prior to diagnosis. However, the results in the Health Professionals Follow-up Study were independent of pre-diagnostic diet, suggesting that men with prostate cancer may alter their prognosis through post-diagnostic dietary choices. We also acknowledge that our study population was composed of primarily Caucasian US male physicians. While this limits the potential for confounding by sociodemographic factors, our results may not be generalizable to populations with different sociodemographic characteristics. Lastly, this was an observational study, and therefore we cannot conclude that there is a causal relation between saturated or vegetable fat intake after diagnosis and risk of death.
Conclusions
After diagnosis of non-metastatic prostate cancer, saturated fat intake was associated with a higher risk of all-cause mortality, while vegetable fat intake was associated with a lower risk. Men who consumed 10 % fewer calories from animal fat and 10 % more calories from vegetable fat after prostate cancer diagnosis had a 44 % lower risk of mortality. Men diagnosed with non-metastatic prostate cancer should replace calories from carbohydrate or animal fat with vegetable fats, such as those found in olive oil and nuts.
References
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, April 2015
Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E (2011) Smoking and prostate cancer survival and recurrence. JAMA 305:2548–2555
Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM (2011) Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 29:726–732
Joshu CE, Mondul AM, Menke A et al (2011) Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 4:544–551
Richman EL, Kenfield SA, Chavarro JE et al (2013) Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Intern Med 173:1318–1326
Epstein MM, Kasperzyk JL, Mucci LA et al (2012) Dietary fatty acid intake and prostate cancer survival in Orebro County. Swed Am J Epidemiol 176:240–252
Strom SS, Yamamura Y, Forman MR, Pettaway CA, Barrera SL, DiGiovanni J (2008) Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer 122:2581–2585
Meyer F, Bairati I, Shadmani R, Fradet Y, Moore L (1999) Dietary fat and prostate cancer survival. Cancer Causes Control 10:245–251
Gaziano JM, Glynn RJ, Christen WG et al (2009) Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 301:52–62
Stampfer MJ, Willett WC, Speizer FE et al (1984) Test of the national death index. Am J Epidemiol 119:837–839
Willett WC (1998) Nutritional epidemiology, 2nd edn. Oxford University Press, Oxford
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC (1992) Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 135:1114–1126
Hunter DJ, Rimm EB, Sacks FM et al (1992) Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 135:418–427
Hu FB, Stampfer MJ, Rimm E et al (1999) Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 149:531–540
Kim H, Yokoyama W, Davis PA (2014) TRAMP prostate tumor growth is slowed by walnut diets through altered IGF-1 levels, energy pathways, and cholesterol metabolism. J Med Food 17:1281–1286
Kim DJ, Gallagher RP, Hislop TG et al (2000) Premorbid diet in relation to survival from prostate cancer (Canada). Cancer Causes Control 11:65–77
Jenkins DJ, Kendall CW, Banach MS et al (2011) Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 34:1706–1711
Hudthagosol C, Haddad EH, McCarthy K, Wang P, Oda K, Sabate J (2011) Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J Nutr 141:56–62
Wien M, Bleich D, Raghuwanshi M et al (2010) Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr 29:189–197
Li Z, Song R, Nguyen C et al (2010) Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr 29:198–203
Kay CD, Gebauer SK, West SG, Kris-Etherton PM (2010) Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr 140:1093–1098
Torabian S, Haddad E, Rajaram S, Banta J, Sabate J (2009) Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet 22:64–71
Razquin C, Martinez JA, Martinez-Gonzalez MA, Mitjavila MT, Estruch R, Marti A (2009) A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur J Clin Nutr 63:1387–1393
Jenkins DJ, Kendall CW, Marchie A et al (2002) Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 106:1327–1332
Castaner O, Covas MI, Khymenets O et al (2012) Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am J Clin Nutr 95:1238–1244
Lopez-Miranda J, Perez-Jimenez F, Ros E et al (2010) Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr Metab Cardiovasc Dis 20:284–294
Estruch R, Ros E, Salas-Salvado J et al (2013) Primary prevention of cardiovascular disease with a mediterranean diet. New Engl J Med 368(14):1279–1290
Demark-Wahnefried W, Polascik TJ, George SL et al (2008) Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomark Prev 17:3577–3587
Luo C, Li Y, Wang H et al (2013) Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr Cancer Drug Targets 13:625–639
Acquaviva R, Di Giacomo C, Sorrenti V et al (2012) Antiproliferative effect of oleuropein in prostate cell lines. Int J Oncol 41:31–38
Park SY, Nho CW, Kwon DY, Kang YH, Lee KW, Park JH (2013) Maslinic acid inhibits the metastatic capacity of DU145 human prostate cancer cells: possible mediation via hypoxia-inducible factor-1alpha signalling. Br J Nutr 109:210–222
Reiter RJ, Tan DX, Manchester LC et al (2013) A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Invest 31:365–373
Davis PA, Vasu VT, Gohil K et al (2012) A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br J Nutr 108:1764–1772
Sanchez-Gonzalez C, Ciudad CJ, Noe V, Izquierdo-Pulido M (2014) Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct 5:2922–2930
Gupta-Elera G, Garrett AR, Robison RA, O’Neill KL (2012) The role of oxidative stress in prostate cancer. Eur J Cancer Prev 21:155–162
Sfanos KS, De Marzo AM (2012) Prostate cancer and inflammation: the evidence. Histopathology 60:199–215
Acknowledgments
This work was supported by grants from the United States Department of Defense (W81XWH-11-1-0529), the National Institutes of Health (CA42182, CA58684, CA90598, CA141298, HL35464, CA167552, CA34944, CA40360, HL26490, HL34595), and the Prostate Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Van Blarigan and Dr. Kenfield contributed equally to this work.
Rights and permissions
About this article
Cite this article
Van Blarigan, E.L., Kenfield, S.A., Yang, M. et al. Fat intake after prostate cancer diagnosis and mortality in the Physicians’ Health Study. Cancer Causes Control 26, 1117–1126 (2015). https://doi.org/10.1007/s10552-015-0606-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0606-4