Abstract
Purpose
The relationship between hormone replacement therapy (HRT) and the incidence of meningioma in women has been investigated in several epidemiologic studies, but their results were not entirely consistent. Here, we performed a meta-analysis of case–control and cohort studies to analyze this association.
Methods
The PubMed database was searched from inception to 30 September 2012 to identify relevant studies that met pre-stated inclusion criteria. We also reviewed reference lists from the retrieved articles. Two researchers evaluated study eligibility and extracted the data independently. Odds ratios (ORs) or relative risks and 95 % confidence intervals (CIs) were extracted and pooled using the fixed-effect or random-effects models.
Results
A total of 11 studies (six case–control and five cohort studies) were included in this meta-analysis, involving 1,820,954 participants, of whom 3,249 had meningioma. When compared to never users of HRT, the pooled OR with ever users for meningioma was 1.29 (95 % CI 1.03–1.60). Sensitivity analyses restricted to postmenopausal women yielded similar results (OR: 1.22; 95 % CI 1.02–1.46). Subgroup analyses showed that the pooled ORs were 1.27 (95 % CI 1.08–1.49, p < 0.05) and 1.12 (95 % CI 0.95–1.32) for current and past users of HRT, respectively.
Conclusion
Hormone replacement therapy use is associated with an increased risk of meningioma in women, as well as in postmenopausal women. Besides, the significant risk elevation is present in current users but not in past users. Future research should attempt to establish whether this association is causal and to clarify its mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningioma is the secondly most common brain tumor, corresponding to an incidence rate of approximately 3.5/100,000 per year worldwide and constituting between 13 and 25 % of all adult primary intracranial tumors [1, 2]. Meningiomas are largely benign and typically slow growing, which generate from the arachnoid cap cells embedded in the arachnoid villi and rarely displaying biologically aggressive behavior [3]. Nowadays, the etiology of meningiomas remains largely unknown. Established risk factors including age, ionizing radiation, and some rare genetic conditions can only explain a small portion of total cases [4, 5]. Many other risk factors such as brain injury, smoking, chronic virus infection, and occupational exposures have been suggested as risk factors, but no definitive conclusions can be drawn.
The incidence of meningiomas is two to three times more common in women than in men, which suggest that hormones could influence the development of meningioma. Increased tumor growth rates have been reported during pregnancy [6], and an association exists between breast cancer and meningioma [7]. Molecular studies show that progesterone and estrogen receptors are present in approximately 70 % and 30 % of meningiomas, respectively [8, 9]. Proliferation of human meningioma cell lines after exposure to estrogen and progesterone has also been observed [10]. On the basis of these observations, there has been much speculation that hormone replacement therapy (HRT) may be a risk factor for meningioma in women.
Many epidemiologic studies have been published from different countries investigating the association of HRT in women and the incidence of meningioma [11–21], but the results are inconsistent. Besides, there is still no systematic and quantitative assessment of published findings on this topic, and we therefore conducted a meta-analysis to better clarify this issue.
Materials and methods
Search strategy
We attempted to report this meta-analysis follow the proposed (meta-analysis of observational studies in epidemiology) MOOSE guidelines [22]. We systematically searched the PubMed databases to identify citations of previously published (up to 30 September 2012) relevant studies using the following search terms: (1) hormone replacement therapy, hormonal exposures, estrogen replacement therapy, reproductive factors; (2) meningioma, brain tumor. In addition, we have searched the reference lists of all identified relevant publications. No language restriction was applied.
Eligibility and criteria
There were two reviewers (Fan and Wu) to evaluate studies independently for possible inclusion and resolve any discrepancy by discussion. The reviewers were blinded to journal and institution. Studies were included if they met the following criteria: (1) used a case–control or cohort study design; (2) investigated the associations between HRT and risk of intracranial meningioma in women; (3) meningioma cases were medically confirmed; (4) provided the odds ratios (OR) or relative risk (RR) with confidence intervals (CIs) or data necessary to calculate them; (5) data including at least one of the following hormone exposure variables were given: ever versus never users, current users, and past users.
Data extraction
The following data were extracted from each of the studies that met the inclusion criteria: study name, authors, publication year, study site, study design, follow-up years for cohort studies, sample size (numbers of case patients and control subjects or cohort size), instruments used for data collection, matching variables, and statistical adjustments for confounding factors. In cases where more than one article was published on the same population of patients, the most recent or most informative report was selected for analysis. ORs or RRs and their respective 95 % CI were either extracted directly from the article or calculated from available raw data.
Statistical analysis
STATA 11 (StataCorp, College Station, TX, USA) was used for all statistical analysis. The measure of effect of interest is the OR with 95 % CI. Because the risk of meningioma is low, the relative risk in prospective cohort studies mathematically approximates the odds ratio [23], therefore permitting the combination of case–control and cohort studies. The multivariate-adjusted ORs and 95 % CI presented in the literature were used. If the OR was not available, raw data were used to calculate it. The potential between-study heterogeneity was assessed using the χ2-based Q statistical test and the I2 test [24, 25]. Heterogeneity was considered significant when I2 was >50 % and p was <0.10. When no heterogeneity existed, the results from the individual studies were combined using the fixed-effects model with the Mantel–Haenszel method [26]. Otherwise, the random-effect model with the DerSimonian–Laird method [25] was used for pooling.
Because HRT is used mostly in postmenopausal women, we further conducted a sensitivity analysis restricted to postmenopausal women to assess the association between HRT and incidence of meningioma.
Publication bias was estimated using the STATA procedure of “Metabias,” which is based on two different approaches, Begg’s [27] and Egger’s tests [28]. p < 0.05 was considered indicative of significant publication bias. All p values were two-sided.
Results
Literature search
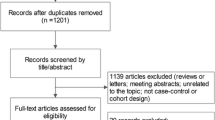
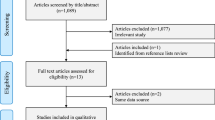
Figure 1 shows a flow diagram of the selection process for relevant studies. Searches of the PubMed generated 647 potentially relevant articles. Of these, the majority were excluded after the first screening based on abstracts or titles. The full texts of 20 of them were further reviewed. Six studies were excluded because they did not investigate the association between HRT and meningioma risk [29–34]. One study was excluded because it was not a comparative study [35]. Two reviews were also excluded [36, 37]. Finally, 11 studies [11–21] were eligible for this meta-analysis.
Study characteristics
The characteristics of the 11 eligible studies are presented in Table 1. These studies were published between 2003 and 2012, involving a total of 1,820,954 participants, of whom 3,249 were meningioma patients. Of the 11 studies, six were case–control studies and five were cohort studies. Five studies were conducted in the USA, two in the UK, one each in Canada, Sweden, Finland, and one was a multi-country study. Among the 11 enrolled studies here, six studies evaluated the association between current/past hormone use and meningioma risk. One study did not adjusted for confounders [13], while the other studies adjusted for some variables, such as age, gender, race, education, body mass index (BMI), smoking status, oral contraceptive use, family history with brain tumors, marital status, and distance from home to hospital.
Overall association of HRT use with the risk of meningioma
The results of the pooled analysis for ever use of HRT are shown in Fig. 2. Among the 11 enrolled studies, four showed a significantly positive relationship between HRT use and the incidence of meningioma. The ORs ranged from 0.70 (95 % CI 0.40–1.20) to 2.20 (95 % CI 1.90–2.60). There was a significant heterogeneity among these studies (I2 = 80.6 %, p < 0.01), and the random-effects model was used to calculate the summary OR. In the pooled analysis, the summary ORs associated with ever HRT users in comparison with never users in the case–control studies were 1.08 (95 % CI 0.88–1.33) and 1.58 (95 % CI 1.20–2.09) in the cohort studies. When the cohort and case–control data were combined, the cumulative OR for all studies was 1.29 (95 % CI 1.023–1.60, p < 0.05), suggesting a significant positive association between HRT use and the risk of meningioma.
Current and past HRT use
The meta-analysis of current and past use of HRT (vs. never) included six studies [11, 12, 14, 18, 19, 21], comprised of three case–control and three cohort studies. No statistically significant heterogeneity was detected among those studies, so the fixed-effects model was used. For current users, the pooled OR was 1.27 (95 % CI 1.08 to 1.49, p < 0.05) (Fig. 3a), which suggested current use of HRT was associated with an increased risk of the occurrence of meningioma. Subgroup analysis showed that the summary ORs were 0.97 (95 % CI 0.75–1.25) in the case–control studies and 1.51 (95 % CI 1.23–1.85) in the cohort studies. Inversely, no association was found between past use of HRT and the risk of meningioma (for all studies: OR: 1.12; 95 % CI 0.95–1.32; for case–control studies: OR: 1.00; 95 % CI 0.79–1.26; for cohort studies: OR: 1.27; 95 % CI 1.00–1.63) (Fig. 3b).
Sensitivity analyses and publication bias
Nine of the studies included in our meta-analysis examined the relation between the use of HRT and the incidence of meningioma in postmenopausal women [11–15, 17–20], so we conducted a sensitivity analysis restricted to postmenopausal women. The pooled OR was 1.22 (95 % CI 1.02–1.46), 1.03 (95 % CI 0.77–1.39), and 1.39 (95 % CI 1.18–1.63) for case–control, cohort, and all studies, respectively, with a middle heterogeneity among these studies (I2 = 50.0 %, p = 0.058).
Begg’s test indicated no publication bias among studies of HRT use and meningioma risk (p = 0.436) (Fig. 4), but Egger’s test indicated a possible publication bias (p = 0.050). Visual inspection of the Begg’s funnel plot did not identify substantial asymmetry. These results suggested a possibility that publication bias may have played a role in the observed effect, but it was unlikely to explain the full magnitude of the association.
Discussion
This is the first systematic review and meta-analysis of published studies assessing the risk of meningioma in women using hormone replacement therapy. Our meta-analysis provided evidence that HRT use is associated with an increased risk of meningioma in women, as well as in postmenopausal women. In addition, the significant risk elevation is present in current users but not in past users.
As meningiomas are rare, the majority of studies investigating risk factors, including the use of HRT, were retrospective case–control studies. Our meta-analysis contains 11 studies with six case–control and five cohort studies. The potential for misclassification and differential reporting of HRT use, a problem in all retrospective studies, is particularly strong for brain tumors, as patient’s memories may have been affected by the tumors. Besides, owing to information about exposure relying on the participants’ memory and proxy interviews, recall bias is complicated commonly. The majority of case–control studies have shown no association between HRT use and the incidence of meningioma, which is contradictory to our finding. On the contrary, one noticeable advantage of the cohort study is that all participants are recruited before meningioma develops, and the collection of data occurs near the exposure. Therefore, the data are more accurate. Our observation for meningioma risk and HRT use was consistent with the two largest prospective cohort studies included in the present meta-analysis: the European Prospective Investigation into Cancer and Nutrition study (ever vs. never users, RR: 1.73; 95 % CI 1.23–2.43; current vs. never users, RR: 1.79; 95 % CI 1.18–2.71) [19] and the Million Women Study (ever vs. never, RR: 1.32; 95 % CI 1.05–1.66; current vs. never, RR: 1.34; 95 % CI 1.03–1.75) [18].
Clinical evidences have been suggested that meningioma might be a hormone-sensitive tumor based on observations of higher incidence in women than in men [1], an observed increased development of meningioma during pregnancy [6] and the luteal phase of menstruation [38, 39], increased incidence of meningioma in women with breast cancer [7], and growth of meningioma after estrogen–progestin therapy in transsexual patients [40, 41]. Biological data support an association as well: in vitro studies have shown proliferation of meningioma cells with exposure to estradiol or progesterone [10]. Estrogen and progesterone receptors have been shown to be expressed in meningioma [8, 9]; however, the significance of these findings is unclear.
The present meta-analysis showed an increased risk of meningioma for current users of HRT in women, and the association among past users was not as strong in our study. It is conceivable that female hormone involves increased tumor growth rates during the exposure period (current use) and that the risk decreases with time after no longer being a current user. However, it is still unclear how hormones are involved in brain tumors from a mechanistic standpoint.
Several limitations of this study should be considered. First, significant heterogeneity was found across our main analysis, which might have resulted from inconsistencies in study designs, sample sizes, analysis strategies, and participants’ baseline characteristics. For example, both case–control studies and cohort studies are included in this meta-analysis. Five of the six case–control studies involved in our meta-analysis are population based [13–15, 17, 21], and only one study is hospital based [12]. Meanwhile, several studies with relatively small numbers of participants were included in the present meta-analysis [12–15], which raised some concerns regarding the reliability of their results. This heterogeneity reduced the statistical power for detecting a possible association between HRT use and meningioma. To address this, however, we used the random-effects model to determine the overall estimate of variability.
Second, we could not obtain information on the main confounders from most studies. One is a history of oral contraceptive use, which may increase the hormone intake in women. Only one study [19] examined whether the association between HRT use and meningioma was modified by oral contraceptive use. Among the enrolled studies, most of the them adjusted for age, four studies adjusted for education [12, 14, 15, 19], three studies adjusted for BMI [11, 18, 19], and one study did not adjusted for confounding factors [13]. Therefore, our results should be considered with some caution because of the potential confounding.
A third limitation was the different methods to assess the participants’ history of HRT use. Assessment instruments to get information of HRT exposure consisted of interview [12, 14, 15, 17] and questionnaires [11, 13, 18, 19, 21]. With different methods, the participants may have different attitudes and may not understand the questions in an identical way. All these could affect the accuracy of the data collection. Besides, the dose of HRT use varied across studies and participants. Therefore, our findings should be considered with some caution because of different assessment methods and dosage of HRT use.
Finally, potential publication bias might influence the findings. The Begg’s test indicated no publication bias, but the Egger’s tests showed a possible publication bias for these studies. Our relatively strict inclusion criteria might have introduced selection bias.
In summary, this meta-analysis is suggestive of an elevated risk of meningioma among ever and current use of HRT in women. In contrast, past use of HRT was not associated with the risk of meningioma. However, considering the limitations of our meta-analysis, our results should be interpreted with caution. Further study is needed to confirm our findings and to explore the underlying mechanisms and elucidate the causal pathways that link female hormone with meningioma.
References
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57(6):1088–1095
Bondy M, Ligon BL (1996) Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 29(3):197–205
Ragel BT, Jensen RL (2005) Molecular genetics of meningiomas. Neurosurg Focus 19(5):E9
Krampla W, Newrkla S, Pfisterer W et al (2004) Frequency and risk factors for meningioma in clinically healthy 75-year-old patients. Cancer 100(6):1208–1212
Phillips LE, Frankenfeld C, Drangsholt M, Koespell TD, vanBelle G, Longstreth WT (2005) Intracranial meningioma and ionizing radiation in medical and occupational settings. Neurology 64(2):350–352
Koper JW, Lamberts SW (1994) Meningiomas, epidermal growth factor and progesterone. Hum Reprod 9(Suppl 1):190–194
Custer BS, Koepsell T, Mueller BA (2002) The association of breast carcinoma and meningioma in women. Cancer 94(6):1626–1635
Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86(1):113–120
Speirs V, Boyle-Walsh E, Fraser WD (1997) Constitutive co-expression of estrogen and progesterone receptor mRNA in human meningiomas by RT-PCR and response of in vitro cell cultures to steroid hormones. Int J Cancer 72(5):714–719
Jay JR, MacLaughlin DT, Riley KR, Martuza RL (1985) Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg 62(5):757–762
Jhawar BS, Fuchs CS, Colditz GA, Stampfer MJ (2003) Sex steroid hormone exposures and risk for meningioma. J Neurosurg 99(5):848–853
Hatch EE, Linet MS, Zhang J et al (2005) Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer 114(5):797–805
Lee E, Grutsch J, Persky V, Glick R, Mendes J, Davis F (2006) Association of meningioma with reproductive factors. Int J Cancer 119(5):1152–1157
Custer B, Longstreth WT Jr, Phillips LE, Koepsell TD, Van Belle G (2006) Hormonal exposures and the risk of intracranial meningioma in women: a population-based case-control study. BMC Cancer 6:152
Wigertz A, Lönn S, Mathiesen T et al (2006) Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol 164(7):629–636
Blitshteyn S, Crook JE, Jaeckle KA (2008) Is there an association between meningioma and hormone replacement therapy? J Clin Oncol 26(2):279–282
Korhonen K, Raitanen J, Isola J, Haapasalo H, Salminen T, Auvinen A (2010) Exogenous sex hormone use and risk of meningioma: a population-based case–control study in Finland. Cancer Causes Control 21(12):2149–2156
Benson VS, Pirie K, Green J et al (2010) Hormone replacement therapy and incidence of central nervous system tumours in the Million Women Study. Int J Cancer 127(7):1692–1698
Michaud DS, Gallo V, Schlehofer B et al (2010) Reproductive factors and exogenous hormone use in relation to risk of glioma and meningioma in a large European Cohort Study. Cancer Epidemiol Biomarkers Prev 19(10):2562–2569
Johnson DR, Olson JE, Vierkant RA et al (2011) Risk factors for meningioma in postmenopausal women: results from the Iowa Women’s Health Study. Neuro-Oncol 13(9):1011–1019
Cea-Soriano L, Blenk T, Wallander MA, Rodríguez LA (2012) Hormonal therapies and meningioma: is there a link? Cancer Epidemiol 36(2):198–205
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127(9):820–826
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Wigertz A, Lonn S, Hall P et al (2008) Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prev 17(10):2663–2670
Schlehofer B, Blettner M, Wahrendorf J (1992) Association between brain tumors and menopausal status. J Natl Cancer Inst 84(17):1346–1349
Lambe M, Coogan P, Baron J (1997) Reproductive factors and the risk of brain tumors: a population-based study in Sweden. Int J Cancer 72(3):389–393
Cantor KP, Lynch CF, Johnson D (1993) Reproductive factors and risk of brain, colon, and other malignancies in Iowa (United States). Cancer Causes Control 4(6):505–511
Benson VS, Pirie K, Green J, Casabonne D, Beral V (2008) Lifestyle factors and primary glioma and meningioma tumours in the million women study cohort. Br J Cancer 99(1):185–190
Schlehofer B, Blettner M, Preston-Martin S et al (1999) Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer 82(2):155–160
Korhonen K, Auvinen A, Lyytinen H, Ylikorkala O, Pukkala E (2012) A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol 175(4):309–314
Cowppli-Bony A, Bouvier G, Rué M et al (2011) Brain tumors and hormonal factors: review of the epidemiological literature. Cancer Causes Control 22(5):697–714
Claus EB, Black PM, Bondy ML et al (2007) Exogenous hormone use and meningioma risk: what do we tell our patients? Cancer 110(3):471–476
Bickerstaff ER, Small JM, Guest IA (1958) The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatr 21(2):89–91
Michelsen JJ, New PF (1969) Brain tumour and pregnancy. J Neurol Neurosurg Psychiatr 32(4):305–307
Gazzeri R, Galarza M, Gazzeri G (2007) Growth of a meningioma in a transsexual patient after estrogen-progestin therapy. N Engl J Med 357(23):2411–2412
Deipolyi AR, Han SJ, Parsa AT (2010) Development of a symptomatic intracranial meningioma in a male-to-female transsexual after initiation of hormone therapy. J Clin Neurosci 17(10):1324–1326
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in the manuscript entitled “Hormone replacement therapy and risk of meningioma in women: a meta-analysis.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, ZX., Shen, J., Wu, YY. et al. Hormone replacement therapy and risk of meningioma in women: a meta-analysis. Cancer Causes Control 24, 1517–1525 (2013). https://doi.org/10.1007/s10552-013-0228-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-013-0228-7