Abstract
Objective
Epidemiological evidences indicate that diabetic individuals may have an increased risk of several cancers; however, the relationships between diabetes and risk of cancers of biliary tract or its subsites remain unclear.
Methods
To provide a quantitative assessment of this relationship, we identified studies by a literature search of Medline (from 1 January 1966) and EMBASE (from 1 January 1974), through 31 July 2010, and by searching the reference lists of pertinent articles. Summary relative risks with corresponding 95% confidence intervals were calculated with a random-effect model.
Results
Analysis of 21 studies (8 case–control and 13 cohort studies) found that diabetes was associated with an increased risk of biliary tract cancer, compared with no diabetes (summary RRs = 1.43, 95% CI = 1.18–1.72), with significant heterogeneity among studies (p = 0.001). The positive association was also found between diabetes and risk of gallbladder cancer or extrahepatic cholangiocarcinoma, but not cancer of ampulla of Vater. No significant publication bias was found.
Conclusion
These findings strongly support the link between diabetes and increased risk of cancer of biliary tract and its subsites: gallbladder cancer or extrahepatic cholangiocarcinoma, but not cancer of ampulla of Vater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary tract cancers (BTCs) include a spectrum of invasive adenocarcinomas: extrahepatic cholangiocarcinoma (ECC), cancer of ampulla of Vater (AVC), and gallbladder cancer (GBC). Every year, about 12,000 people were afflicted by BTC in the United States [1], and in Shanghai, China, a rapid increase was also seen in the incidence of BTC over the last three decades [2]. Additionally, incidence rates of GBC and ECC, the two common components of BTC, have different variation trends: the incidence rates of GBC are increasing, whereas the rates of ECC have been steady [3]. From a general viewpoint, prognosis of BTC is very poor, and surgical resection is associated with 5-year survival rates of 24–40% [4]. However, only about 20% of patients are fortunate enough to undergo resection of their tumors at the time of diagnosis [5]. To explore the effective tools for the prevention of BTC, identification of epidemiologic factors that influence the development of BTC would facilitate prevention and/or early detection of this disease.
Unfortunately, due to the rarity and high fatality of BTC or cancer of its subsites, little is known about their etiologies. To date, several risk factors, such as obesity, history of gallstones or cholecystitis, and lifestyle-related factors, have been suggested for GBC and ECC development [6–9]. However, most individuals who exposed to these known environmental risk factors never develop these cancers; on the other hand, quite a number of these cancers could develop among individuals without exposure to these risk factors.
The relationship between diabetes mellitus (DM) and malignancies has long been investigated. Among these studies, the most consistently reported associations are the associations between DM and risk of liver cancer [10] and pancreatic cancer [11]. The hypothesized biological mechanisms are related to the effect of insulin and insulin-like growth factors (IGFs) on cellular growth and anti-apoptosis, which are involved in the development and progression of malignancies [12, 13]. With respect to the association of DM and risk of BTC or cancer of its subsites, most studies are compatible with a positive association. To provide a quantitative assessment of the relationship between DM and risk of BTC and cancer of its subsites, we conducted a meta-analysis as a systematic approach to review published studies evaluating the association between DM and risks of BTC and cancer of its subsites. We also evaluated whether the associations varied by sex and by study design.
Materials and methods
Data sources and searches
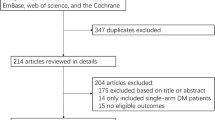
A computerized literature search was conducted in Medline (from 1 January 1966) and EMBASE (from 1 January 1974), through 31 July 2010, by two independent investigators (H. Ren and T. Yu). We searched the relevant studies with the following text word and/or Medical Subject Heading (MeSH) terms: “diabetes mellitus,” “diabetes,” “biliary tract cancer,” “extrahepatic cholangiocarcinoma,” “gallbladder cancer,” “cholangiocarcinoma,” “cancer of ampulla of Vater,” “epidemiologic studies.” Furthermore, we reviewed citations from retrieved articles to search for more studies. No language restrictions were imposed.
Study selection
Studies were included in this systematic review if (1) they had a case–control or cohort design; (2) the interests of outcome were BTC, AVC, ECC, and/or GBC; (3) relative risk (RR) in cohort studies or odds ratio (OR) in case–control studies and their 95% confidence intervals (CIs) (or data to calculate them) were reported. If data were duplicated in more than one study, the most recent study was included in the systematic review. This resulted in the exclusion of one article from our systematic review [14]. Articles or reports from non-peer-reviewed sources were also not considered for this analysis.
Data extraction
Two researchers independently extracted the following data from each publication: the first author’s last name, year of publication, country where the study was performed, study design, ascertainment of interest of exposure and outcome, sample size (cases and controls or cohort size), variables adjusted for in the analysis, and the RR estimates with corresponding 95% CIs. From each study, we extracted the risk estimates that reflected the greatest degree of control for potential confounders. If studies reported both incidence rate and mortality rate, we extracted the incidence rate, since mortality rate could be confounded by survival-related factors.
Statistical analysis
We included in this systematic review reporting different measures of relative risks: case–control studies (OR), cohort studies (rate ratio, hazard ratio), and cohort studies of diabetic patients using external population comparisons (standardized incidence ratio, SIR). In practice, these measures of effect yield similar estimates of RR because the absolute risk of BTC or cancer of its subsites is low. We did not include the study with standardized mortality ratio (SMR) [15] in the calculation of a summary measure.
Summary relative risk estimates with their corresponding 95% CIs were calculated with the methods of DerSimonian and Laird by the use of the assumptions of a random-effects model that considers both within- and between-study variation [16]. If studies that reported relative risks specific for both sexes, respectively, we calculated a pooled RR and its corresponding 95% CI. If the association between DM and risk of cancer of subsites of biliary tract were reported together, we first analyzed together (as RR estimates for BTC) and then separately (as RR estimates for cancer of its subsites). Statistical heterogeneity among studies was evaluated using the Q and I 2 statistics [17]. For the Q statistic, a p value of less than 0.10 was considered representative of statistically significant heterogeneity. I 2 is the proportion of total variation contributed by between-study variation.
We conducted analyses stratified by study design, gender, and geographic area, and we also evaluated the impact of adjustment for smoking and body mass index (BMI) on the association between diabetes and the risk of BTC or GBC. To assess the potential publication bias, we used a funnel plot by the Begg’s adjusted rank correlation test and by the Egger’s regression test [18, 19]. All statistical analyses were performed with STATA, version 11.0 (STATA, College Station, TX, USA). A two-tailed p value < 0.05 was considered to be significant.
Results
Study characteristics
There were 21 studies included in this systematic review (Tables 1, 2). The continents or countries in which the studies were conducted were as follows: Asia (n = 8), the United States (n = 7), and Europe (n = 6).
The eight case–control studies reported a total of 1,874 cases with BTC or cancer of its subsites; among these 1,874 cases, 320 patients with diabetes were reported (Table 1), whereas among 165,861 control subjects, a total of 25,387 subjects having diabetes were reported. Control subjects recruited were originated from a population-based [7, 20, 21] or hospital-based setting [8, 22–25]. Diabetes status was ascertained by self-reported history of DM or hospital records in all studies. Diagnosis of BTC or cancer of its subsites was based on histological methods in four studies [8, 22, 23, 25] and on a review of medical records or diagnostic codes in three studies [7, 20, 24]. Ascertainment of outcome was based on histological methods or medical records in another one study [21]. Adjustments were made for potential confounders of one or more factors in seven of eight studies, with the exception of one study [8].
We identified 13 prospective cohort studies that reported an association between diabetes and risk of BTC or cancer of its subsites (Table 2). Among these 13 studies, eight were cohort studies, using incidence and/or mortality rate ratios as the measure of relative risk, and five were diabetic cohorts, using SIR/SMR as the measure of relative risk. The former eight cohorts included 5,218,193 subjects; among them, 1,054,564 subjects with diabetes, and the latter diabetic cohorts included 276,782 participants (range: 1,135–153,852) with diabetes. These 13 cohort studies had a median duration of follow-up of 10 years (range: 2.3–20 years), reporting a total of 2,493 incident cases of BTC or cancer of its subsites. The methods of DM ascertainment were on the basis of self-reported history of DM or blood glucose levels test in most studies, except for two studies in which the ascertainment of DM was based on registry of disease [26, 27]. The ascertainment of outcome was based on cancer registry in all studies, with the exception of one study in which GBC diagnosis was made by histological evaluation [28]. Potential confounders were controlled in all studies.
Diabetes and risk of BTC
We identified two case–control studies [21, 23] and seven cohort studies [27, 29–34] that presented results on diabetes and risk of biliary tract cancer. Another diabetic cohort study reported results with SMR [15]. Of these, five studies did not find an increased risk of BTC in patients with diabetes [21, 23, 31–33], and in another five studies, positive relationships were found [15, 27, 29, 30, 34].
In analysis of all these nine studies that reported relative risk of DM and BTC, the summary RRs and corresponding 95% CIs were 1.43 (95% CI 1.18–1.72) in a random-effects model for those with diabetes compared with those without diabetes. There was statistically significant heterogeneity among studies (Q = 22.71, p = 0.001, I 2 = 71.1%) (Fig. 1).
We then conducted subgroup meta-analyses by sex, geographic area, and study design (Table 3). Six studies provided results on cancer incidence stratified by sex and found that diabetic males had an increased risk of developing BTC (summary RRs = 1.31; 95% CIs 1.17–1.47; test for heterogeneity, Q = 2.72, p = 0.743, I 2 = 0), which was similar in diabetic females (summary RRs = 1.29; 95% CIs 1.13–1.46; test for heterogeneity, Q = 3.74, p = 0.442, I 2 = 0% vs. males, z = 0.18, p = 0.86). In addition, summary RRs of the associations between diabetes and BTC risk were significantly higher for studies conducted in non-Asia (the United States and Europe) than in Asia [summary RRs (95% CI), 1.66(1.26–2.19) vs. 1.23 (1.11–1.37); z = 1.99, p = 0.047]. Lastly, a positive association between DM and BTC risk was found in cohort studies (summary RRs = 1.42; 95% CIs 1.16–1.74), and a positive, but non-significant association was found in case–control studies (summary RRs = 1.50; 95% CIs 0.72–3.13).
Body mass index (BMI) and smoking status are two of the most important confounders for the positive association between diabetes and BTC risk. When we limited the meta-analysis to the four studies that controlled for BMI [21, 32–34], a positive association but not significant between diabetes and BTC risk was found (summary RRs = 1.46; 95% CIs 0.88–2.42, test for heterogeneity Q = 6.30, p = 0.098, I 2 = 52.4%). Similarly, when the meta-analysis was restricted to the studies controlled for smoking [31–34], an increased but not significant risk of BTC was also found for history of DM (summary RRs = 1.39; 95% CIs 0.89–2.19, test for heterogeneity Q = 17.78, p < 0.001, I 2 = 83.1%).
Diabetes and risk of cancer of subsites of biliary tract
DM and GBC risk
Individual study results and the summary results for four case–control studies [20, 21, 24, 35] and seven cohort studies [28, 29, 33, 34, 36–38] of diabetes and GBC risk are shown in Fig. 2. Three of these eleven studies found a statistically significant positive association between diabetes and GBC risk (range of individual RRs, 0.57–2.63; summary RRs for all 11 studies 1.52, 95% CIs 1.26–1.84). There was no significant heterogeneity among studies (p = 0.141, I 2 = 32.2%).
Subgroup meta-analyses by study design indicated that the positive association was statistically significant among both cohort studies (summary RRs = 1.49, 95% CIs: 1.19–1.86, p = 0.129 for heterogeneity) and case–control studies (summary RRs = 1.63, 95% CIs: 1.07–2.47, p = 0.254 for heterogeneity) (Table 3). One case–control study [21] and five cohort studies [28, 29, 33, 36, 37] presented results specific for sex, and we found an increased risk of GBC in diabetic females (vs. non-diabetic females) but not in diabetic males (vs. non-diabetic males). The association between diabetes and GBC risk was significantly positive in studies conducted in non-Asia (summary RRs = 1.48, 95% CI: 1.19–1.85, p = 0.107 for heterogeneity), but not in Asia (summary RRs = 1.61, 95% CI: 0.98–2.62, p = 0.194 for heterogeneity).
When we limited the meta-analysis to studies that controlled for BMI [20, 21, 24, 33, 34, 36], a positive association between diabetes and GBC risk was found (summary RRs 1.65, 95% CIs 1.22–2.23, p = 0.083 for heterogeneity). Lastly, when restricting the meta-analysis to the studies that controlled for smoking [20, 24, 29, 33, 34, 36], we found an increased risk of GBC in diabetic individuals, compared with those with no diabetes (summary RRs 1.45, 95% CIs 1.16–1.82, p = 0.082 for heterogeneity).
Diabetes and risk of ECC
We identified four case–control studies [7, 8, 21, 25] and three cohort studies [26, 29, 34] that presented results on diabetes and ECC risk, and the summary RRs of ECC were 1.59 (95% CI 1.26–1.99; p = 0.011 for heterogeneity) in a random-effects model for those with diabetes compared with those without diabetes (Fig. 3). Because only one study reported results specific for gender [29], we stratified data into subgroups according to study design and geographic area. A positive association was found among both cohort studies (summary RRs, 1.54; 95% CI 1.06–2.23) and case–control studies (summary RRs, 1.62; 95% CI 1.05–2.49). In addition, the summary estimates were significant for studies conducted in non-Asia (summary RRs 1.55, 95% CI 1.24–1.95; p = 0.022 for heterogeneity) but not in Asia (summary RRs 1.62, 95% CI 0.74–3.57; p = 0.038 for heterogeneity) (Table 3).
Diabetes and risk of AVC
Two studies [21, 29] presented results on the association between DM and AVC risk (data not shown). One [29] reported a statistically significant positive association, and the other [21] observed a non-statistically significant association. When the two studies were pooled, a positive, but non-significant association between diabetes and AVC risk was found (summary RRs 1.60, 95% CI 0.60–4.22, test for heterogeneity p = 0.146).
Publication bias
The funnel plot revealed no evidence for publication bias concerning diabetes and risk of BTC and cancer of its subsites. p Values for Begg’s adjusted rank correlation test and Egger’s regression asymmetry test were 0.61 and 0.82, respectively, both suggesting that publication bias probably has little effect on summary estimates (Fig. 4).
Discussion
This review indicates that compared with non-diabetic individuals, diabetic individuals (largely type two diabetes) may have an approximately 50% increased risk of developing BTC and similarly increased risk for ECC or GBC is also found. Nevertheless, we find no significant association between diabetes and AVC risk.
To date, this is the first systematic review to comprehensively and systematically evaluate the observational studies that report a link between type 2 DM and risk of cancer of bile tract or its subsites. In the present study, we included a large number of studies; thus, we could assess the association according to sex and cancer subsites with high precision, avoiding insufficient statistical power to detect some relationships in the individual studies due to small sample sizes.
Although the absolute risks of BTC are low among diabetic individuals, our results have important clinical and public health significance. This is due to the following reasons. Initially, DM is a very common disease; in the past two decades, the prevalence of DM has been elevated markedly in both developed countries and developing countries [39, 40]. Second, the incidence and mortality rates of BTC have been on the rise worldwide, although malignancies of the biliary tract are less common. For example, in Shanghai, China, biliary tract cancer was the most rapidly increasing malignancy from 1972 to 1994, with an increase in incidence of 119% in men and 124% in women [41].
There are several potential limitations that should be considered. First, case–control studies are susceptible to recall and selection biases; cohort studies might be affected by detection bias because patients with diabetes are under increased medical surveillance and thus might be more likely to be diagnosed as BTC. These biases may distort the true effects. Second, most of the studies included in this manuscript did not distinguish between type 1 and type 2 diabetes. So, some degree of misclassification of exposure to diabetes is likely to have occurred. This non-differential misclassification would tend to attenuate the magnitude of the association between diabetes and BTC risk. However, it is likely that the vast majority of individuals with diabetes included in these studies had type 2 diabetes, because this disease is by far the most common form particularly in older individuals. Third, confounding is also likely to be present,because history of DM may also reflect other factors related to an unhealthy lifestyle, such as smoking, heavy alcohol consumption, and obesity. Such unhealthy lifestyles have generally been associated with an increased risk of cancer. Although most studies controlled for these lifestyle factors, the possibility of residual confounding cannot be completely excluded. Thus, the observed increased risk of BTC associated with a history of diabetes may reflect confounding by these risk factors. However, a positive association between diabetes and BTC or gallbladder cancer risk remained when we limited the meta-analysis to studies that controlled for BMI or smoking. Finally, as in any meta-analysis, the possibility of publication bias is of concern, because small studies with null results tend not to be published. However, the results obtained from funnel plot analysis and formal statistical tests did not provide evidence for such bias.
A relationship between diabetes and risk of bile tract cancer is biologically plausible. Type 2 diabetes is associated with insulin resistance, compensatory hyper-insulinemia, and up-regulated level of insulin-like growth factors (IGFs). IGFs may stimulate growth through cellular proliferation and inhibition of apoptosis within the cholangiocytes [42]. An important role of IGFs in the carcinogenesis of cholangiocytes is supported by in vitro and in vivo studies [43]. Additionally, in some, but not all, studies, diabetes has been independently associated with an increased risk of biliary stones [21, 44], which are one of the major risk factors for BTC, especially for GBC. In the study from China, Shebl et al. [21] found that diabetes was associated with a twofold risk of biliary stones, and about 60% of the effect of diabetes on biliary tract cancer was mediated by gallstones. The authors also found that diabetes was related to several key factors important in the process of stone formation [21, 45]. In line with these investigation, Biddinger et al. [46] also found that insulin resistance might result in increased production of biliary cholesterol and lithogenic bile salt, which directly promoted the formation of gallstones.
Type 2 diabetes and bile tract cancer or gallbladder cancer share similar risk factors, including cigarette smoking and obesity(BMI ≥ 30) [8, 47]. So, the observed positive associations between these cancers and a history of diabetes may reflect confounding by the two risk factors. When we limited the meta-analysis to studies which were adjusted for the two risk factors, a positive association between diabetes and GBC risk remained. However, the magnitude of the association between diabetes and BTC risk has been attenuated and is not significant. This indicated an effect of confounding may be existed.
By subgroup analysis, significantly positive associations between diabetes and risk of GBC and ECC were found for studies conducted in non-Asia but not in Asia. Similarly, summary RRs of the associations between diabetes and BTC risk were significantly higher for studies conducted in non-Asia than in Asia (p = 0.047). These results may reflect the differences in ethnic and lifestyle between the West and the East.
In summary, the results from this systematic review strongly support an association between diabetes and increased risks of cancer of biliary tract or its subsites in both women and men. However, the possibility that the association may be due to bias or confounding cannot be ruled out. More research, both epidemiological and mechanistic, is needed to further clarify the association between diabetes and risk of cancer of bile tract or its subsites.
Abbreviations
- BTC:
-
Biliary tract cancer
- GBC:
-
Gallbladder cancer
- ECC:
-
Extrahepatic cholangiocarcinoma
- AVC:
-
Cancer of ampulla of Vater
- DM:
-
Diabetes mellitus
- AORs:
-
Adjusted odds ratios
- CI:
-
Confidence intervals
- IGFs:
-
Insulin-like growth factors
- SIR/SMR:
-
Standardized incidence/mortality ratio
- HR:
-
Hazard ratio
References
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24:115–125
Jin F, Devesa SS, Chow WH et al (1999) Cancer incidence trends in urban shanghai, 1972–1994: an update. Int J Cancer 83:435–440
Shaib YH, Davila JA, McGlynn K, El-Serag HB (2004) Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 40:472–477
Seyama Y, Makuuchi M (2007) Current surgical treatment for bile duct cancer. World J Gastroenterol 13:1505–1515
Jarnagin WR, Fong Y, DeMatteo RP et al. (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234:507–517; discussion 517–509
Larsson SC, Wolk A (2007) Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer 96:1457–1461
Welzel TM, Graubard BI, El-Serag HB et al (2007) Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case–control study. Clin Gastroenterol Hepatol 5:1221–1228
Shaib YH, El-Serag HB, Nooka AK et al (2007) Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case–control study. Am J Gastroenterol 102:1016–1021
Welzel TM, Mellemkjaer L, Gloria G et al (2007) Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case–control study. Int J Cancer 120:638–641
El-Serag HB, Hampel H, Javadi F (2006) The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 4:369–380
Ben Q, Cai Q, Li Z et al (2011) The relationship between new-onset diabetes mellitus and pancreatic cancer risk: a case–control study. Eur J Cancer 48:248–254
Frasca F, Pandini G, Sciacca L et al (2008) The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 114:23–37
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591
Adami HO, McLaughlin J, Ekbom A et al (1991) Cancer risk in patients with diabetes mellitus. Cancer Causes Control 2:307–314
Verlato G, Zoppini G, Bonora E, Muggeo M (2003) Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 26:1047–1051
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Grainge MJ, West J, Solaymani-Dodaran M, Aithal GP, Card TR (2009) The antecedents of biliary cancer: a primary care case–control study in the United Kingdom. Br J Cancer 100:178–180
Shebl FM, Andreotti G, Rashid A et al (2010) Diabetes in relation to biliary tract cancer and stones: a population-based study in Shanghai, China. Br J Cancer 103:115–119
La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P (1994) A case–control study of diabetes mellitus and cancer risk. Br J Cancer 70:950–953
Khan ZR, Neugut AI, Ahsan H, Chabot JA (1999) Risk factors for biliary tract cancers. Am J Gastroenterol 94:149–152
Kuriki K, Hirose K, Tajima K (2007) Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev 16:83–89
Tao LY, He XD, Qu Q et al (2010) Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case–control study in China. Liver Int 30:215–221
El-Serag HB, Engels EA, Landgren O et al (2009) Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of US veterans. Hepatology 49:116–123
Chen HF, Chen P, Li CY (2010) Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology 52:155–163
Ragozzino M, Melton LJ III, Chu CP, Palumbo PJ (1982) Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis 35:13–19
Adami HO, Chow WH, Nyren O et al (1996) Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst 88:1472–1477
Wideroff L, Gridley G, Mellemkjaer L et al (1997) Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 89:1360–1365
Jee SH, Ohrr H, Sull JW et al (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293:194–202
Inoue M, Iwasaki M, Otani T et al (2006) Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166:1871–1877
Khan M, Mori M, Fujino Y et al (2006) Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan collaborative cohort (JACC) study. Asian Pac J Cancer Prev 7:253–259
Jamal MM, Yoon EJ, Vega KJ, Hashemzadeh M, Chang KJ (2009) Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J Gastroenterol 15:5274–5278
La Vecchia C, Negri E, Decarli A, Franceschi S (1997) Diabetes mellitus and the risk of primary liver cancer. Int J Cancer 73:204–207
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159:1160–1167
Yagyu K, Lin Y, Obata Y et al (2004) Bowel movement frequency, medical history and the risk of gallbladder cancer death: a cohort study in Japan. Cancer Sci 95:674–678
Swerdlow AJ, Laing SP, Qiao Z et al (2005) Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer 92:2070–2075
Yang W, Lu J, Weng J, et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362:1090–1101
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
Hsing AW, Gao YT, Devesa SS, Jin F, Fraumeni JF Jr (1998) Rising incidence of biliary tract cancers in Shanghai, China. Int J Cancer 75:368–370
Cai HH, Sun YM, Bai JF et al (2008) Relationship between the GH-IGFs axis and the proliferation of bile duct cancer cell line QBC939 in vitro. Hepatobiliary Pancreat Dis Int 7:76–81
Alvaro D, Barbaro B, Franchitto A et al (2006) Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol 169:877–888
Festi D, Dormi A, Capodicasa S et al (2008) Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 14:5282–5289
Stone BG, Van Thiel DH (1985) Diabetes mellitus and the liver. Semin Liver Dis 5:8–28
Biddinger SB, Haas JT, Yu BB et al (2008) Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 14:778–782
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578
Conflict of interest
There are no potential conflicts of interest among all authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, HB., Yu, T., Liu, C. et al. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control 22, 837–847 (2011). https://doi.org/10.1007/s10552-011-9754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-011-9754-3