Abstract
We investigated whether MC1R genotype modifies the effect of sun exposure on melanoma risk in 1,018 cases with multiple melanomas (MPM) and 1,875 controls with one melanoma (SPM). There was some suggestion that MC1R genotype modified the effect of beach and water activities on MPM risk: ORs were 1.94 (95% CI 1.40–2.70) for any activities for no R variants and 1.39 (95% CI 1.05–1.84) with R variants (R151C, R160W, D294H, and D84E) (p for interaction 0.08). MC1R modification of sun exposure effects appeared most evident for MPM of the head and neck: for early life ambient UV, the OR was 4.23 (95% CI 1.76–10.20) with no R and 1.04 (95% CI 0.40–2.68) with R (p for interaction = 0.01; p for three-way interaction = 0.01). Phenotype modified the effect of sun exposure and MPM in a similar manner. We conclude that MC1R and pigmentary phenotype may modify the effects of sun exposure on melanoma risk on more continuously sun-exposed skin. Possible explanations include that risk may saturate with higher sun sensitivity for melanomas on continuously sun-exposed sites but continue to increase as sun exposure increases with lower sun sensitivity, or that sun-sensitive people adapt their behavior by increasing sun protection when exposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People who have had one melanoma have an increased risk of a second. The Genes, Environment and Melanoma (GEM) study population includes cases who had a second or higher order melanoma and controls who had a first primary incident melanoma. We have found that risk of a subsequent or multiple primary melanoma (MPM) increased with increasing ambient UV irradiance at places of residence and with lifetime recreational exposure, particularly in beach and waterside activities. The results for ambient UV were very consistent with evidence from studies of individual risk of any primary melanoma in relation to place of birth and age at migration from or to areas of high ambient UV irradiance [1, 2]. The increased risk for recreational sun exposure was likewise consistent with the findings of Gandini et al’s meta-analysis [3].
Melanoma is mainly a disease of light-skinned populations. The chief pigmentary traits associated with melanoma risk are fair skin, blonde or red hair, blue eyes, a skin that sunburns easily, tans poorly, and freckles. These are highly correlated traits, however, and are known to be partially determined by the melanocortin 1 receptor, MC1R [4, 5], some variants of which result in a defect in activation of melanin production [6]. Almost all GEM participants (90%) had MC1R genotypes measured: cases were more likely than controls to carry multiple and higher-risk MC1R variants [7].
In this paper, we examine whether variants in MC1R and pigmentary phenotypes modify the relationship between sun exposure and risk of subsequent melanoma in GEM and, if so, what the patterns in exposure–response relationships by genotype were.
Methods
GEM included as participants all incident cases of melanoma notified to 8 population-based cancer registries in Australia (2 registries), Canada (2), Italy (1), and the USA (3) in a defined accrual period; participants from a Michigan center were excluded from this report because of incomplete data on sun exposure and related covariates. Detailed methods have been described elsewhere [7–9]. Briefly, controls were diagnosed with a first invasive primary melanoma in 2000 and cases with a second or higher-order invasive or in situ melanoma in 2000–2003. Inclusion of in situ cases was designed to avoid exclusion of subjects who would have been diagnosed with an invasive subsequent primary if the lesion had not been detected in the in situ stage. All participants provided written informed consent, and approval for the study protocol was obtained from Institutional Review Boards at the GEM Coordinating Center, the Memorial Sloan-Kettering Cancer Center, New York, and each contributing center.
All participants completed a self-administered calendar and questionnaire before a 1-h telephone interview and gave a buccal DNA sample [10]. Lifetime residential histories were recorded in the calendar and used for assigning levels of ambient UV exposure. The questionnaire asked for skin, hair, and eye color in standard categories. The interview included questions on ethnicity, ancestry, propensity to burn on first sun exposure in summer, tanning ability on repeated sun exposure, and sun exposure in decade years from 10 years of age.
Ambient solar UV irradiance for each participant was estimated as the annual average erythemally weighted UV at their place of residence at birth and in each decade of age. These values were model based and supplied to GEM by the US National Center for Atmospheric Research (Dr Julia M Lee Taylor). To calculate the lifetime average annual UV irradiance for each participant, the value for UV irradiance at birth (age 0) was assigned to each year from birth to age 4, that at age 10 to each year from age 5 to age 14, and so on, using irradiance in the last decade year up to the exact age at diagnosis and dividing the total by age. Early life ambient UV was calculated as the average of UV irradiance at birth and age 10.
Recreational sun exposure in beach and water activities from age 15 was obtained from direct questions about participation between 9 a.m. and 5 p.m. on at least 10 days in any year since leaving school, the years started and stopped, the frequency, usual outdoor hours per day, and the seasons; a lifetime sum of hours was calculated. Although vacations in a sunnier climate and sunburns were included initially in the present analyses because of their known associations with MPM [9], we excluded them from this report because they appeared to add little information beyond the contribution of beach and water activities and ambient UV.
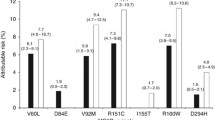
DNA was isolated from buccal cells and MC1R sequenced for 2,202 controls and 1,099 cases in the GEM study (90% of participants), in whom 85 unique variants were identified [7]; 88% of participants in the centers included in this report had MC1R genotyped. The current analyses follow Kanetsky et al.’s [7] grouping in which people with two copies of the consensus wild type (con/con) were the reference category, and there were two additional categories, one formed by grouping people with any red hair–associated (R) variant (R151C, R160W, D294H, D84E) in an ‘any R’ category (con/R, r/R, R/R) and those having only other variants into an ‘r’ category (con/r; r/r). For most analyses, we examined MC1R variants in 2 categories as no R (con/con, con/r; r/r) and any R variants (con/R, r/R, R/R). Carriage of synonymous variants did not affect group assignment. When we examined the association of each MC1R variant with red hair, ORs were 3.0 or higher for each of 7 variants: the 4 R variants R151C, R160W, D294H, and D84E had p values <0.01 and Y152X, 86insA, and 537insC had p values <0.09 and had been previously identified as significantly associated with melanoma risk [11] or likely to produce a non-functional transmembrane receptor [12]. The latter 3 variants were included in the R genotype in sensitivity analyses.
We included only participants who reported exclusively European ancestry because of the known phenotypic differences in other ethnic groups and their small numbers in GEM. Included in these analyses are 1,018 cases and 1,875 controls (2,893 GEM participants) who had MC1R variants genotyped, of whom 75 participants developed their first primary in the control accession period and their second primary in the case accession period. Since epidemiologic theory clearly indicates that these participants should be included as both cases and controls in the analysis [13], they were included as both [10].
Statistical analyses
Conventional methods for case–control studies were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for occurrence of an MPM in unconditional logistic regression models in SAS software version 9.1 (SAS Institute, Cary NC, 1989). Individuals with missing data for a variable were excluded from the relevant analysis. Two-sided p values were calculated. Study center was included as a covariate in all regression models in this analysis, and all were adjusted for a priori confounders: age (continuous; age at first melanoma diagnosis for controls, age at most recent for cases), sex, European ancestry in 6 categories, and an age * sex interaction term [9]. A GEM-wide pigment score was calculated using a multivariate confounder score [14, 15] to summarize the contribution of ability to tan, propensity to burn, skin, eye, and hair color, and childhood freckling. Beta-values for each of these factors were estimated using a single unconditional logistic regression model, with case–control status as the outcome. A summation score for each participant was then calculated using these beta-values. Skin color made the largest contribution to fit of the model. An individual’s pigment score was included in analyses as a continuous variable, a higher score indicating a more sun-sensitive phenotype. Site of the melanoma was examined in 2 categories: melanoma on the head and neck (more sun exposed), trunk and limbs (less sun exposed).
A multiple variable logistic regression model was used to identify each sun exposure variable that was independently associated with MPM risk: it included MC1R and sun exposure variables with p ≤ 0.05 for their associations with MPM, and age, sex, age * sex, center, ancestry, and pigment score as covariates. To test for statistical interaction between MC1R genotype and phenotype or sun exposure, MC1R genotype was treated as a binary variable (no R variant, any R variant), and all pigmentary and sun exposure variables were dichotomized. The effects of two-way interactions on MPM risk were evaluated on a multiplicative scale for MC1R and sun exposure, phenotype and sun exposure, and MC1R and phenotype. ORs were calculated with reference to the joint low-exposure category for main effect terms for each of the variables (e.g., genotype and exposure) and the cross product interaction term. The likelihood ratio test was used to assess departure from lack of interaction, comparing a model with main effects to a model with main effects and the interaction term. To generate p values for differences in two-way interactions by site of the melanoma, we compared a model with the main effects, all two-way product terms, and all relevant covariates to a corresponding model with the three-way product term, e.g., MC1R by sun exposure by body site.
Results
The majority of GEM participants in these analyses (2,431 of 2,893, 84%) carried at least one MC1R variant. Consistent with a previous report from GEM [7], carriage of any R variant (R151C, R160 W, D294H, and D84E) relative to none (con/con or r in the absence of R) had ORs of 1.45 (95% CI 1.22–1.72), 1.43 (95% CI 1.20–1.71) for just one R variant and 1.53 (95% CI 1.14–2.07) for two or more; adjusted for age, sex, center, ancestry, and age * sex interaction (Table 1). With reference to the con/con genotype, the ORs were 1.02 (95% CI 0.78–1.34) for any r variant in the absence of R (con/r or r/r) and 1.47 (95% CI 1.14–1.89) for any R (p for trend <0.001) (not tabulated).
ORs for MPM by phenotype were lowest for poor tanning ability, tendency to burn and any childhood facial freckling, intermediate for red hair, relative to other colors, and highest for fair skin and for the most sun-sensitive pigment score, Q4; all p values were <0.05 (Table 1). Relative to black hair, the OR for red hair was 2.05 (95% CI 1.26–3.34), for blond or fair was 1.58 (95% CI 1.02–2.45), for light brown hair was 1.46 (95% CI 0.95–2.25), and for dark brown was 1.14 (0.74–1.78) (p for trend = 0.001). Eye color had no apparent effect on MPM risk. All models for phenotype were adjusted for age, sex, center, ancestry, and age * sex interaction.
The strongest associations of sun exposure with MPM were for any beach and waterside activities and >median average annual lifetime ambient UV (Table 1). In a multivariable model, any beach and waterside activities (OR = 1.58; 95% CI 1.25–1.99) and having any R genotype (OR = 1.34; 95% CI 1.11–1.62) were both statistically significantly associated with MPM (p < 0.05) with pigment score, age, sex, center, ancestry, and age * sex interaction included as covariates. The p values were >0.05 for addition individually to this model of the variables for ambient lifetime and early life UV. The ORs changed very little when the R genotype included the 3 variants Y152X, 86insA, and 537insC in addition to R151C, R160W, D294H, and D84E.
There was some evidence that MC1R genotype modified the effects of beach and water activities (Table 2). The OR for MPM was higher for any activities in the absence of R variants (OR = 1.94) than in their presence (OR = 1.39) (p for interaction 0.08) (Table 2). Adding the 3 variants Y152X, 86insA, and 537insC to the 4 variants R151C, R160W, D294H, and D84E changed the ORs only slightly. There was little evidence of any similar modification of the effects of lifetime or early life ambient UV (Table 2).
We further examined the modification of the association of sun exposure with MPM by MC1R genotype in two body site categories. The ORs for ambient early life UV were higher in those with no R (OR = 4.23) than in those with any R (OR = 1.04) for melanomas on the head and neck (p = 0.01 for two-way interaction) and significantly different by body site (p = 0.01 for three-way interaction), with no evidence of a similar interaction for MPM at other body sites. Lifetime ambient UV had a similar pattern of ORs, but all p values for interaction were high. The higher OR of MPM with beach and water activities in those with no R variants was mostly for MPM on body sites other than the head and neck but not significantly different by body site (p = 0.89 for three-way interaction) (Table 3).
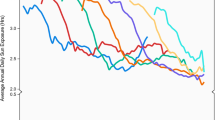
Risk for MPM of the head and neck increased strongly with increasing lifetime ambient UV across 4 exposure categories, and the OR for Q4 was 5.30 (95% CI 1.44–19.58; p for trend 0.01); for early life UV, it was 4.19 (95% CI 1.83–9.63; p for trend <0.001). For other body sites, ORs for ambient UV rose only to 1.75 and showed no apparent trend. The increase in ORs for MPM on the head and neck with increasing lifetime ambient UV was evident only in the absence of MC1R R variants. In people with no R variants, the ORs for Q4 were 14.61 for lifetime ambient UV, 7.8 for early life ambient UV, and 2.75 for beach and water activities, compared with ORs <2.0 when R variants were present (see Fig. 1). There was little evidence of these divergent patterns of increasing MPM risk with increasing ambient UV for melanomas of other body sites. For beach and water activities, MPM risk did not increase consistently beyond the second exposure quarter (OR ~ 2), and there was little evidence that the pattern of increase differed by body site or by MC1R genotype (Fig. 1).
Risk of MPM on head and neck and other body sites: ORs for quartiles of average annual lifetime ambient UV (a, b) and average early life UV (c, d), and ORs for beach and water activities (none, tertiles of hours of average annual lifetime activities) (e, f) by MC1R genotype (no R, any R) in analyses adjusted for age, sex, age * sex, center, ancestry, pigment score; and ORs for beach and water activities (none, tertiles of hours of average annual lifetime activities) by pigmentary phenotype (sun resistent, sun sensitive) (g, h) in analyses adjusted for age, sex, age * sex, center, and ancestry
Like MC1R genotype (Table 3), phenotype also appeared to modify the effects of sun exposure on risk of MPM of the head and neck (Table 4). This effect was most evident for beach and water activities: the OR was 3.74 in those with a pigment score less than or equal to the median and 0.87 in those with a pigment score greater than the median score (p = 0.04 for three-way interaction) (Table 4). The OR estimates in Table 4 changed little when MC1R genotype (no R, any R) was added to the model. The ORs in 4 quarters of beach and water activities for MPM of the head and neck rose with increasing exposure to Q3 in the less sun sensitive; ORs for the more sun sensitive were low (see Fig. 1).
There was no consistent pattern of modification of effects of phenotype by MC1R genotype: p for interaction >0.20 for each of skin color, hair color, freckling, ability to tan, and pigment score (results not shown). It may be noteworthy, though, that the positive association of red hair with MPM was restricted to those with any R variants: OR = 1.35 (95% CI 1.00–1.83) for red hair with reference to other hair colors in those with any R variants compared with OR = 0.60 (95% CI 0.18–1.98) in those with no R variants (p for interaction = 0.21). Similarly, when the associations of all hair colors with MPM were examined with reference to black hair, the ORs in those with any R were all appreciably greater than unity: ORs of 1.75 (95% CI 0.80–3.83) for dark brown hair, 1.66 (95% CI 0.78–3.57) for light brown, 1.97 (95% CI 0.91–4.25) for blonde or fair, and 2.37 (95% CI 1.08–5.21) for red hair. The highest OR in those with no R was 1.32 (95% CI 0.78–2.23) for light brown hair (p for interaction 0.86).
Discussion
We observed a moderately consistent pattern of increasing risk of melanoma with increasing sun exposure in people who had no MCIR R variants and thus had lower sun sensitivity. This pattern was confined to early life ambient UV and melanomas on the head and neck. Phenotype appeared to modify the effects of sun exposure on melanoma risk in a manner similar to MC1R genotype, but for beach and water activities only. There was no consistent evidence of genotype–phenotype interaction apart from some suggestion that a positive association of red hair with melanoma might be restricted to people with R variants.
Our findings are consistent with a report on sun exposure modification of MC1R effects in which risk of melanoma for R variants was increased threefold for lower sun exposure but not all for higher exposure [16]. Other studies found no evidence of modification of MC1R effects by sun exposure [17–19] or did not report on sun exposure separately [20–22]. No study apart from our own has directly examined MC1R genotype modification of sun exposure effects.
In the few studies exploring how phenotype modifies the effects of sun exposure, melanoma risk with high sun exposure was greater with more sun-sensitive (summary ORs 2.4–4.0) than less sun-sensitive phenotypes (summary ORs 1.1–1.5) [23]. In our data, an increase in melanoma risk with higher sun exposure was generally more evident in the less sun-sensitive phenotype, especially on the more continuously sun-exposed sites of the head and neck (Table 4), the opposite of the pattern in the earlier studies [23]. Our findings, however, are supported by more recent studies reporting a greater relative risk of melanoma with cumulative time outdoors in those who usually develop a deep tan than in those who do not [24], a stronger association between sunbed use and melanoma in people with dark than light or red hair [25] and that severe sunburn was associated with an increased risk of melanoma for a sun-resistant but not a sun-sensitive phenotype (Cust et al., unpublished data).
That the effect of pigmentary phenotype appeared independent of MC1R genotype suggests that other genes that determine pigmentary or sun sensitivity phenotype also modify sun exposure effects on melanoma risk. Other gene variants consistently associated with pigmentary phenotype [26, 27] and with melanoma [28–31] may have such a role. There are, however, no reports in which the effects of the interactions of other gene variants with sun exposure on melanoma risk have been studied.
If our findings were confirmed in future studies, it would suggest that MC1R or other sun sensitivity genotypes and pigmentary phenotype are modifiers of the effects of sun exposure on risk of melanoma, especially on more continuously sun-exposed skin. Further, it could suggest that the relative increase in sun exposure within the observed ranges on risk of melanoma is greater with lower than higher sun sensitivity. One potential explanation may be a ‘saturation’ effect in which genotype or phenotype modifies the risk associated with high doses of UV exposure. Under this model, more sun-sensitive people may reach a limit beyond which sun exposure does not increase their risk further, while those less sensitive show a continuing rise in relative risk across the observed range of exposures. That effect modification was observable on the head and neck may suggest the effect was limited to one of the possible causal pathways to melanoma under the divergent pathway hypothesis, a pathway in which high levels of more continuous cumulative sun exposure drive the proliferation of epidermal melanocytes to cancer [32].
Differences in sun protection behavior between more-sensitive and less-sensitive people are another possible explanation. People with sun-sensitive phenotypes adapt their behavior to make greater use of sun protection when exposed [33], an adaptive behavior that might flatten the increase in melanoma risk with increasing sun exposure. It may occur before or after, and possibly as a consequence of, a first diagnosis of sun-related neoplasia or pre-neoplasia [23].
We also observed that the associations of red and other fairer hair colors with melanoma may be confined to people with MC1R variants. This observation was unexpected. Adding the red hair phenotype to its main genetic determinants should have had little additional effect on melanoma risk. It was consistent, however, with addition of risk of melanoma by the R151C MC1R variant (which is strongly correlated with red hair) in red haired women [20].
It is important to consider that some observed associations or interactions may have arisen by chance, given the number of gene–environment combinations that we explored and that the p values for interaction were not very low. On the other hand, that findings in other studies [24, 25] [Cust et al., unpublished data] were in the same direction as ours argues against it being due simply to chance. The GEM study relied on self-reported pigmentary phenotype and sun exposure history, which could produce both non-differential and differential misclassification and lead to bias. Differential recall bias, however, would be minimized for the measurements of ambient UV irradiance at places of residence used in our analyses, since they offer a largely objective measure of potential for sun exposure at each decade of age. In addition, differential recall depending on a complex mix of case or control status, sun sensitivity, and melanoma site would be required to produce the patterns we observed. Finally, studies on the reproducibility of standard sun exposure questions suggest reasonable reliability of participant responses [34, 35] and agreement with histologic assessment of solar skin damage [36]. Other strengths in GEM were population-based ascertainment of participants, that participation was unlikely to be related to MC1R genotype since observed genotype frequencies were in the range of previous studies (see Kanetsky [7]) and that relative risk estimates in GEM for skin type, skin color, eye color, and recreational sun exposure were consistent with a meta-analysis [3, 9, 37, 38].
Ours is the first substantial examination of MC1R genotype and pigmentary phenotype modification of the effect of sun exposure on risk of melanoma. Our findings offer suggestive evidence that the effect of sun exposure on melanoma risk for the more continuously exposed head and neck sites may be modified by MC1R genotype and by pigmentary phenotype. Risk of melanoma rose the most with increasing sun exposure in people without R variants in MC1R or with less sun-sensitive phenotypes. These findings should be replicated in studies that are large enough to demonstrate them with much greater certainty, if present, before a model of the interaction of sun exposure and genotype or phenotype based on them can be suggested with any confidence. Pooled analyses involving multiple studies with relevant data would be informative.
References
Holman CD, Armstrong BK (1984) Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst 72(2):257–266
Kaldor J, Khlat M, Parkin DM, Shiboski S, Steinitz R (1990) Log-linear models for cancer risk among migrants. Int J Epidemiol 19(2):233–239
Gandini S, Sera F, Cattaruzza MS et al (2005) Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 41(1):45–60
Rees JL (2003) Genetics of hair and skin color. Annu Rev Genet 37:67–90
Sturm RA, Teasdale RD, Box NF (2001) Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene 277(1–2):49–62
Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA (2005) Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet 14(15):2145–2154
Kanetsky PA, Rebbeck TR, Hummer AJ et al (2006) Population-based study of natural variation in the melanocortin-1 receptor gene and melanoma. Cancer Res 66(18):9330–9337
Begg CB, Orlow I, Hummer AJ et al (2005) Lifetime risk of melanoma in CDKN2A mutation carriers in a population-based sample. J Natl Cancer Inst 97(20):1507–1515
Kricker A, Armstrong BK, Goumas C et al (2007) Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes Control 18(3):295–304
Begg CB, Hummer AJ, Mujumdar U et al (2006) A design for cancer case-control studies using only incident cases: experience with the GEM study of melanoma. Int J Epidemiol 35(3):756–764
Galore G, Azizi E, Scope A, Pavlotsky F, Yakobson E, Friedman E (2007) The Y152X MC1R gene mutation: occurrence in ethnically diverse Jewish malignant melanoma patients. Melanoma Res 17(2):105–108
Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA (2008) Red hair is the null phenotype of MC1R. Hum Mutat 29(8):E88–E94
Rothman KJ, Greenland S (1998) Modern epidemiology. Lippencott-Raven, Philadeplhia
Cook EF, Goldman L (1989) Performance of tests of significance based on stratification by a multivariate confounder score or by a propensity score. J Clin Epidemiol 42(4):317–324
Miettinen OS (1976) Stratification by a multivariate confounder score. Am J Epidemiol 104(6):609–620
Landi MT, Kanetsky PA, Tsang S et al (2005) MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J Natl Cancer Inst 97(13):998–1007
Matichard E, Verpillat P, Meziani R et al (2004) Melanocortin 1 receptor (MC1R) gene variants may increase the risk of melanoma in France independently of clinical risk factors and UV exposure. J Med Genet 41(2):e13
Fargnoli MC, Altobelli E, Keller G, Chimenti S, Hofler H, Peris K (2006) Contribution of melanocortin-1 receptor gene variants to sporadic cutaneous melanoma risk in a population in central Italy: a case-control study. Melanoma Res 16(2):175–182
Kanetsky PA, Panossian S, Elder DE et al (2010) Does MC1R genotype convey information about melanoma risk beyond risk phenotypes? Cancer
Han J, Kraft P, Colditz GA, Wong J, Hunter DJ (2006) Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer 119(8):1976–1984
Dwyer T, Stankovich JM, Blizzard L et al (2004) Does the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype? Am J Epidemiol 159(9):826–833
Stratigos AJ, Dimisianos G, Nikolaou V et al (2006) Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. J Invest Dermatol 126(8):1842–1849
Armstrong BK (2004) How sun exposure causes skin cancer: an epidemiological perspective. In: Hill D, Elwood JMJ, English DR (eds) Prevention of skin cancer. Kluwer, Dordrecht
Fears TR, Bird CC, Guerry D et al (2002) Average midrange ultraviolet radiation flux and time outdoors predict melanoma risk. Cancer Res 62(14):3992–3996
Westerdahl J, Ingvar C, Masback A, Jonsson N, Olsson H (2000) Risk of cutaneous malignant melanoma in relation to use of sunbeds: further evidence for UV—a carcinogenicity. Br J Cancer 82(9):1593–1599
Han J, Kraft P, Nan H et al (2008) A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet 4(5):e1000074
Nan H, Kraft P, Qureshi AA et al (2009) Genome-wide association study of tanning phenotype in a population of European ancestry. J Invest Dermatol 129(9):2250–2257
Gudbjartsson DF, Sulem P, Stacey SN et al (2008) ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet 40(7):886–891
Bishop DT, Demenais F, Iles MM et al (2009) Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet 41(8):920–925
Nan H, Kraft P, Hunter DJ, Han J (2009) Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer 125(4):909–917
Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW (2010) Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol 130(2):520–528
Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC (2003) Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst 95(11):806–812
Lucas RM, Ponsonby AL, Dear K et al (2009) Associations between silicone skin cast score, cumulative sun exposure, and other factors in the Ausimmune Study: a multicenter Australian study. Cancer Epidemiol Biomarkers Prev 18(11):2887–2894
English DR, Armstrong BK, Kricker A (1998) Reproducibility of reported measurements of sun exposure in a case–control study. Cancer Epidemiol Biomarkers Prev 7(10):857–863
Kricker A, Vajdic CM, Armstrong BK (2005) Reliability and validity of a telephone questionnaire for estimating lifetime personal sun exposure in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 14(10):2427–2432
Karagas MR, Zens MS, Nelson HH et al (2007) Measures of cumulative exposure from a standardized sun exposure history questionnaire: a comparison with histologic assessment of solar skin damage. Am J Epidemiol 165(6):719–726
Gandini S, Sera F, Cattaruzza MS et al (2005) Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer 41(14):2040–2059
Begg CB, Hummer A, Mujumdar U et al (2004) Familial aggregation of melanoma risks in a large population-based sample of melanoma cases. Cancer Causes Control 15(9):957–965
Acknowledgments
The study was conducted by the GEM Study Group: Marianne Berwick (PI University of New Mexico), Memorial Sloan-Kettering Cancer Center: Colin B Begg (Co-PI), Irene Orlow (Co-Investigator), Klaus Busam (Dermatopathologist). Study centers included the following: The University of Sydney and Cancer Council New South Wales, Sydney, Australia: Bruce K Armstrong (PI), Anne Kricker (Co-PI); Menzies Centre for Population Health Research, University of Tasmania, Hobart, Australia: Terence Dwyer (PI, currently at the Murdoch Childrens Research Institute, Melbourne, Victoria), Paul Tucker (Dermatopathologist), Alison Venn (PI); British Columbia Cancer Agency, Vancouver, Canada: Richard P. Gallagher (PI); Cancer Care Ontario, Toronto, Canada: Loraine D. Marrett (PI), Elizabeth Theis (Co-Investigator), Lynn From (Dermatopathologist); Centro per la Prevenzione Oncologia Torino, Piemonte, Italy: Stefano Rosso (PI); Roberto Zanetti (Co-PI); University of California, Irvine: Hoda Anton-Culver (PI); University of Michigan, Ann Arbor: Stephen B. Gruber (PI); University of North Carolina, Chapel Hill: Robert C. Millikan (PI), Nancy Thomas (Co-Investigator); University of Pennsylvania: Timothy R. Rebbeck (PI), Peter Kanetsky (Co- Investigator). UV data consultants: Dr Julia Lee Taylor and Dr Sasha Madronich, National Center for Atmospheric Research, Boulder, Colorado.
Financial support
National Cancer Institute (NCI) grants U01 CA83180 and R01 112524; NCI Preventive Oncology Academic Award grant K07 CA80700 (Peter Kanetsky); University of Sydney Medical Foundation Program grant (Bruce Armstrong); NCI grant CA098438 (Colin. Begg); Michael Smith Foundation for Health Research Infrastructure Award (Richard Gallagher); Lineberger Comprehensive Cancer Center Core grant P30 CA16086 (Robert Millikan).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kricker, A., Armstrong, B.K., Goumas, C. et al. MC1R genotype may modify the effect of sun exposure on melanoma risk in the GEM study. Cancer Causes Control 21, 2137–2147 (2010). https://doi.org/10.1007/s10552-010-9633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-010-9633-3