Abstract
Background
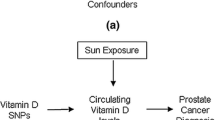
Presence of xenotropic murine leukemia virus–related virus and chronic inflammation in prostate tumor suggests that inflammation plays a role in prostate cancer etiology. This study investigated whether variants in inflammatory genes act alone or interact with plasma antioxidants to influence prostate cancer risk in a population-based case–control study in Central Arkansas.
Methods
Cases (n = 193) were men, aged 40–80, diagnosed with prostate cancer in three major hospitals in 1998–2003, and controls (n = 197) were matched to cases by age, race, and county of residence.
Results
After adjustment for confounders, polymorphisms in COX-2 (rs689466) and IL-8 (rs4073) were not significantly associated with prostate cancer risk. However, apparent interactions were observed between these genetic variants and plasma antioxidants on the risk of this malignancy. The protective effect of the mutant allele of the COX-2 polymorphism was more pronounced among subjects with high plasma levels of β-cryptoxanthin, lycopene, β-carotene, or selenium (≥median) [e.g., OR (95% CI): 0.37 (0.15, 0.86) (AG/GG vs. AA) for β-cryptoxanthin]. Conversely, the promoting effect of the variant allele of the IL-8 polymorphism was more remarkable in subjects with low plasma levels of Lutein/zeaxanthin, β-cryptoxanthin, and β-carotene (<median) [e.g., OR (95% CI): 2.44 (1.08, 5.75) (AT/TT vs. AA) for β-carotene].
Conclusions
We found that sequence variants in inflammatory genes interact with plasma antioxidants to modulate prostate cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is one of the major cancers in Western countries, and its incidence is rapidly increasing in some Asian countries (e.g., China, Korea, and Japan) [1]. In the United States, prostate cancer is the most common cancer and the second leading cause of cancer death in men [2]. The etiology of prostate cancer remains largely unclear. Like many other chronic diseases, prostate cancer may occur as a consequence of the interaction between gene and environment [3, 4]. However, most studies to date have employed a simplistic approach of evaluating either single environmental or genetic factors to examine the complex causes of this disease.
Several lines of evidence suggest that inflammation plays a role in prostate cancer etiology. Chronic inflammation is frequently present in prostate biopsy and prostatectomy specimens [5]. Recently, xenotropic murine leukemia virus–related virus (XMRV) has been identified in malignant prostate epithelium and found to be integrated into human genome DNA extracted from prostate tumor tissue [6]. Furthermore, epidemiologic studies have shown that prostatitis [7] and sexually transmitted infections (e.g., syphilis, gonorrhea) [8] are associated with an increased risk of prostate cancer, but the opposite effect was observed for regular use of nonsteroidal anti-inflammatory drugs [9]. Under chronic inflammatory conditions, inflammatory cells release oxygen-reactive species and thereby enhance oxidative stress [10]. If not counteracted by exogenous antioxidants and endogenous antioxidant enzymes, oxidative stress induces oxidative DNA damage (e.g., 8-OH-dG) that could initiate prostate carcinogenesis [11, 12]. Therefore, it is possible that sequence variants in inflammatory genes act alone or interact with plasma antioxidants to influence prostate cancer risk. The present study investigated this hypothesis in a population-based case–control study in Arkansas.
Materials and methods
Study subjects
A case–control study was conducted in Arkansas to evaluate dietary intake of meat, techniques for cooking meat, and genetic variability in metabolism of carcinogens derived from meat cooked at high temperature in relation to prostate cancer. The design and methods of this study have been described in detail elsewhere [13, 14]. Briefly, cases were patients, aged 40–80, who were diagnosed with primary, incident, and pathologically confirmed prostate cancer during the period 1998–2003. Subjects were excluded from the study if they had a history of cancer (other than nonmelanoma skin cancer), uncontrolled cardiovascular diseases, hepatic dysfunction, or renal dysfunction. Cases were ascertained from the University Hospital of the University of Arkansas for Medical Sciences in Little Rock, the Central Arkansas Veterans Health Care System (CAVHCS) in Little Rock, and the Jefferson Regional Medical Center in Pine Bluff. All these hospitals are major medical centers in central Arkansas where approximately half of all cases of prostate cancer occurring in this area were diagnosed and treated. All cases were recruited to the study within 3 months of diagnosis.
Eligibility criteria for controls were the same as those for cases, disallowing diagnosis of prostate cancer. Controls were randomly selected from the source population of cases and were matched to cases by age (±5 years), race, and county of residence. Specifically, controls were identified from the Arkansas State Drivers’ License records, Centers for Medicare and Medicaid records, and a mass-mailing database, which accounted for 19, 13, and 68% of the total number of controls recruited, respectively. The mass-mailing database contained contact information for approximately 89% of Arkansas residents. Over the period of 5 years, 618 cases and 403 controls were enrolled to the study, with a response rate of 69% for cases and of 56% for controls [13].
The protocol of this study was approved by the appropriate institutional review boards, and informed consent was obtained from all subjects. In-person interview was carried out by trained research assistants at the home of participants or any other place of their preference. A questionnaire tailored to the study population was used to elicit information on demographics, socioeconomics, family cancer history, occupational history, physical activity, cigarette smoking, alcohol drinking, and diet [13].
The present study reported the results obtained from 193 cases and 197 controls that were randomly drawn (for economic reasons) from the 618 cases and 403 controls recruited in the parent study. For this subset of the study subjects, data on genotyping of candidate genes and plasma concentrations of antioxidants were available for testing our hypothesis.
Laboratory measurement
At the end of the interview, a 30-ml blood sample was drawn into tubes containing citric acid, delivered on dry ice to the CAVHCS hospital, and processed within 2 h of collection. Lymphocytes were isolated and plasma was separated from blood. Plasma specimens were then aliquoted into 0.5-ml straws of a sealed capillary tube straw system (CryoBioSystems, Paris, France) and stored in liquid nitrogen tanks at −196°C until analysis [13].
Genotyping
DNA was exacted from peripheral blood lymphocytes using a commercial kit (Qiagen Inc., Valencia, CA). The polymorphisms genotyped in this study were cyclooxygenase-2 (COX-2) (rs689466), lipoxygenase-12 (LOX12) (rs1126667), tumor necrosis factor-α (TNF-α) (rs1800629), interleukin-1β (IL-1β) (rs16944), IL-6 (rs1800795), IL-8 (rs4073), and IL-10 (rs1800871) (Table 2). The selected polymorphisms lie in genes involved in inflammation, have functional impact (e.g., altered expression or associated with a cancer), and are common (>5%) in African Americans or Caucasians. Genotyping of these genetic variants was conducted at BioServe Biotechnologies Ltd. (Beltsville, MD) by high-throughput chip-based matrix-assisted laser desorption time-of-flight mass spectrometry (Sequenom, Inc., San Diego, CA) [14]. A variety of quality control measures were taken to ensure the validity of genotyping data. For example, all laboratory personnel were blinded to the case–control status of DNA specimens, and a 10% of the tested samples were randomly replicated and were found to be 100% concordant [14].
Plasma antioxidants
Plasma samples were packed on dry ice and shipped to the Biomarker Analysis and Lipoprotein Research Laboratories of Harvard School of Public Health for measuring carotenoids and α-tocopherol and to the primary testing center (St. Louis, MO) of the Laboratory Corporation of America for evaluating selenium. Plasma concentrations of α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein/zeaxanthin, and α-tocopherol were determined by high-performance liquid chromatography. Because lutein and zeaxanthin co-elute on the chromatogram, they were grouped and reported as lutein/zeaxanthin. The method, internal and external quality control measures, and between-run and within-run coefficients of variation for the determination of these antioxidants have been described in detail elsewhere [15]. Plasma levels of selenium were measured by inductively coupled plasma-mass spectrometry.
Statistical analysis
To evaluate potential genotyping errors, genotyping data among controls were examined by testing their deviation from the Hardy–Weinberg equilibrium. Unconditional logistic regression analysis was used to estimate odds ratios (OR), and 95% confidence intervals (CI) for prostate cancer risk in relation to genotypes of the polymorphisms considered. In the regression models, subjects who were homozygous for the wild-type allele were treated as the reference group to calculate ORs for those who were heterozygous or homozygous for the variant allele. Heterozygous and homozygous variant genotypes were combined in all analyses because of the relatively small sample size of the present study. To evaluate the independent effects of each of the polymorphisms of interest on prostate cancer risk, age, race, body mass index (weight in kg/height in m2), education (three levels), and smoking status (never, former, and current) were adjusted as confounders in the regression models.
To evaluate the potential interactions between plasma levels of carotenoids, α-tocopherol, and selenium and the genetic variants examined on prostate cancer risk, an interaction term between each of the selected polymorphisms (divided into two genotype groups) with each of the measured antioxidants (classified into< and ≥median) was introduced into the aforementioned multivariable models. The statistical significance of each of the interaction terms constructed was evaluated by the likelihood ratio test. As stated previously, it is biologically plausible that sequence variants in inflammatory genes interact with antioxidants to modulate prostate cancer risk. Therefore, stratified analyses were performed by low and high plasma concentrations of the antioxidants examined (defined as< and ≥median) for the associations between selected polymorphisms and prostate cancer risk regardless of whether the multiplicative interaction terms that were put into the full models were statistically significant. All data analyses were conducted using the SAS software (version 9.1; SAS Institute, Inc., Cary, NC). Statistical significance level was set at p < 0.05 (two-sided).
Results
Characteristics of the study subjects were shown in Table 1. The genetic variants of interest in relation to prostate cancer risk were displayed in Table 2. All polymorphisms were in Hardy–Weinberg equilibrium. After adjustment for age, race, body mass index, education, and smoking status, none of the seven polymorphisms examined was statistically associated with risk, although marginally significant associations were observed for COX-2 (rs689466) [OR (95% CI): 0.64 (0.38, 1.06) (AG/GG vs. AA)] and IL-8 (rs4073) [OR (95% CI): 1.59 (0.96, 2.66) (AT/TT vs. AA)]. No significant interaction was present between polymorphisms in COX-2 (rs689466) and IL-8 (rs4073).
However, apparent interactions were detected between each of these two variants and plasma concentrations of some of the measured antioxidants on prostate cancer risk. Specifically, the protective effect of the variant allele of COX-2 (rs689466) was more pronounced among individuals with high plasma levels (≥median) of all seven antioxidants considered except lutein/zeaxanthin (Table 3). Significantly reduced risk for the AG/GG genotype when compared with the AA genotype [(OR (95% CI)] was found for subjects who had high plasma concentrations of β-cryptoxanthin [(0.37 (0.15, 0.86)], lycopene [(0.41 (0.18, 0.89)], β-carotene [(0.48 (0.23, 0.97)], and selenium [(0.45 (0.22, 0.95)]. Conversely, the promoting effect of the mutant allele of IL-8 (rs4073) was more marked among individuals with low plasma levels (<median) of all seven antioxidants examined except lycopene and selenium (Table 4). Adjusted odds ratios (95% CI) (AT/TT vs. AA) were statistically significant for subjects who had low plasma concentrations of lutein/zeaxanthin [(2.13 (1.03, 4.48)], β-cryptoxanthin [(2.15 (1.07, 4.41)], and β-carotene [(2.44 (1.08, 5.57)].
Whether the associations between the selected genetic polymorphisms and prostate cancer risk differed by race and Gleason score was also evaluated in our analysis. Significantly reduced risk with the variant allele of COX-2 (rs689466) [OR (95% CI): 0.48 (0.24, 0.95)] and significantly increased risk with the variant allele of TNF-α (rs1800629) [OR (95% CI): 1.98 (1.02, 3.95)] and IL-8 (rs4073) [OR (95% CI): 2.41 (1.10, 5.48)] were confined to European Americans. It appeared that the effects of the sequence variants considered on prostate cancer risk were not related to Gleason score (<7 vs. ≥7), with an exception of the IL-8 polymorphism [OR (95% CI): 1.99 (1.10, 3.65) for Gleason score <7 and 1.12 (0.58, 2.18) for Gleason score ≥7]. Three-way interactions between polymorphisms, antioxidants, and race (or Gleason Score) were not evaluated because of inadequate statistical power.
Discussion
In the present study, we observed that polymorphisms in inflammatory genes interacted with plasma concentrations of antioxidants to modulate prostate cancer risk. The favorable effect of the variant allele of COX-2 (rs689466) was more pronounced among individuals with high levels of β-cryptoxanthin, lycopene, β-carotene, and selenium, whereas the detrimental effect of the mutant allele of IL-8 (rs4073) was more remarkable among subjects with low levels of lutein/zeaxanthin, β-cryptoxanthin, and β-carotene.
Pathological, virological, and epidemiological studies suggest that inflammation is implicated in pancreatic carcinogenesis [6, 10, 16]. Therefore, it is reasonable to speculate that sequence variants in genes coding proinflammatory [interleukin-1B (IL-1B), IL-6, IL-8, and tumor necrosis factor-α (TNF-α)] and anti-inflammatory (IL-10) cytokines influence prostate cancer risk [17]. These genetic effects are biologically plausible because polymorphisms in cytokine genes may lead to an altered production of cytokines [18]. Chronic inflammation promotes oxidative stress [19]. Oxidative DNA damage induces carcinogenesis in various human organs including the prostate [11, 12]. It is thus likely that genetic variability in the inflammatory pathway act synergistically with circulating antioxidants to alter pancreatic cancer risk. To date, however, only a few studies have evaluated variants in cytokine genes and no studies have examined interactions of these variants with antioxidants in relation to the risk of prostate cancer.
COX-2 converts arachidonic acid to prostaglandins, potent mediators of inflammation [20]. This enzyme is expressed in the prostate tumor and its increased expression in prostate tumor has been linked to local chronic inflammation and prostate cancer progression [20]. A polymorphism in the promoter of COX-2 (-1195 A/G, rs689466) was found to have functional impact because it creates a c-MYB binding site [21]. The -1195A allele was associated with an enhanced transcriptional activity of COX-2 in a reporter gene assay compared with the -1195G allele [21]. A case–control study conducted in Cleveland, Ohio evaluated the effect of this genetic variant on prostate cancer risk [20]. No significant association was found in that study. In our study, we observed that individuals who carried one or two copies of the -1195G allele experienced a 36% lower risk of prostate cancer than those who only harbored the -1195A allele, although this risk reduction was marginally statistically significant. Our results were consistent with the functionality of this genetic variant. We are not aware of any other studies examining its influence on prostate cancer susceptibility.
IL-8 is involved in inflammatory response by promoting in vitro endothelial cell proliferation and chemotaxis [22]. Its expression regulates angiogenesis, tumorigenicity and metastases in prostate cancer [23, 24]. The role of IL-8 in prostate carcinogenesis gains further support from the observation that serum concentrations of IL-8 were elevated in patients with prostate cancer [25]. The polymorphism in IL-8 (-251A/T, rs4073) was evaluated in relation to prostate cancer in a British study [17] and in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial [26]. A significantly reduced risk was associated with the TT genotype of IL-8 in the British study. However, no apparent effect was detected in the PLCO study. We found that the subjects who carried the -251T allele of IL-8 tended to have a higher risk of prostate cancer than those who harbored the -251A allele. The discrepant results of these studies may be in part due to differences in sample size, subject recruitment, and confounding adjustment. The functional significance of this sequence variant remains inconclusive, although one study suggested that the -251A allele was associated with an insignificantly increased production of IL-8 in whole blood stimulated by lipopolysaccharide [27].
A novel finding of the present study is that interactions between genetic variability in the inflammation pathway and plasma antioxidants may play a role in prostate cancer etiology. Significantly reduced risk for the G allele of COX2 polymorphism (rs699466) and significantly increased risk for the T allele of IL8 polymorphism (rs4073) were more remarkable among subjects with high or low levels of some carotenoids or selenium, respectively. As antioxidants that are abundant in vegetables and fruits, plasma levels of some carotenoids (i.e., lycopene, lutein/zeaxanthin, and β-cryptoxanthin) have been inversely associated with prostate cancer risk in this study population [28]. These gene–antioxidant interactions are biologically plausible because chronic inflammation leads to oxidative stress, a condition that promotes carcinogenesis and is counteracted by antioxidants [11, 12]. This observation may provide new insight into the prevention of prostate cancer in that lowering its risk by increasing dietary intake of antioxidants appears to be particularly relevant and important for individuals who carry risk alleles of inflammatory genes.
In addition to the aforementioned two sequence variants in COX-2 and IL-8, we also investigated polymorphisms in five other inflammatory genes [LOX-12 (rs1126667), TNF-α (rs1800629), IL-1β (rs16944), IL-6 (rs1800795), and IL-10 (rs1800871)]. Neither statistically significant effect of these genetic variants nor appreciable interactions of their interactions with plasma antioxidants were detected in relation to prostate cancer risk. No other studies have examined the relation between the LOX-12 polymorphism and prostate cancer, but this polymorphism has been linked to an elevated risk of esophageal cancer in a Chinese study [29]. The variants in IL-1β and IL-10 were evaluated in the PLCO study, with overall null results [26]. For the TNF-α polymorphism, a significantly increased risk [OR (95%): 1.61 (1.09–2.64)] was found for the AG/AA genotypes in comparison with the GG genotype in a Spanish population [30]. However, a similar effect was not detected in a case–control study nested in the PLCO study and the American Cancer Society cohort study [31]. Like the present study, four other studies [26, 32–34] also revealed an insignificantly promoting effect of the IL-6 polymorphism on prostate cancer risk, although this effect reached statistical significance for the CG genotype [OR (95%): 1.49 (1.05–2.11)] in the Cardiovascular Health Study [33]. Of note, an insignificant protective effect of the IL-6 polymorphism was observed in one study [35]. Because only a few studies have evaluated the effect of genetic variability in inflammatory response on prostate cancer susceptibility, more studies are warranted to better elucidate the role of inflammation in the etiology of this disease.
To our knowledge, this is the first study to evaluate the interactions between polymorphisms in inflammatory genes and plasma levels of antioxidants in relation to prostate cancer risk. Dietary intake of antioxidants assessed by food frequency questionnaire in most epidemiologic studies is subject to recall bias [36]. We overcome this methodological drawback by measuring plasma concentrations of carotenoids, vitamin E, and selenium, which were objective integrated indicators of intake of these nutrients from diet and supplements. Our study subjects consisted of both European and African Americans, which enabled us to evaluate the race-specific effects of polymorphisms in inflammatory genes on prostate cancer. We also investigated whether genetic variability in the inflammation pathway exerts different effects on slow- and fast-growing prostate tumors (evaluated by Gleason score) [37], although such a difference was not observed for all polymorphisms examined except that of IL-8.
There are several weaknesses in the present study. The response rates of this study were relatively low, which might have led to a selection bias and consequently influenced the validity and generalization of our results. Genotyping error needs to be considered in any genetic association studies. However, it should not be a problem in our study because of consistency of all genotyped polymorphisms with the Hardy–Weinberg equilibrium among control subjects and concordance of genotyping results of 10% replicated samples randomly selected from all subjects. In this study, only one polymorphism was selected from each of the seven genes of interest, which suggests that we might have captured a limited amount of sequence variability in these candidate genes. Plasma antioxidants were measured only once, and patients might have changed their dietary habits after the diagnosis or treatment of prostate cancer. Therefore, it was possible that plasma antioxidants for some patients were not well representative of their usual circulating concentrations of these nutrients, which might have introduced misclassification error and in turn resulted in attenuated risk estimates. Plasma levels of antioxidants were determined 3–8 years after blood samples were collected and stored at −196°C. The degradation of these chemical compounds during storage should not be substantial because it was found that plasma levels of β-carotene, retinol, and α-tocopherol were stable for at least 15 years of storage at −70°C [38]. The relatively small sample size of and the number of performed statistical tests in our study made it difficult for us to rule out that some of our results, especially those of stratified analyses, were chance findings. As a result, the detected patterns of interactions between the sequence variants in COX2 and IL-8 and some of plasma antioxidants in relation to prostate cancer risk should be interpreted cautiously and need to be replicated in large epidemiologic studies.
In summary, our study suggested that sequence variants in COX-2 and IL-8 significantly interacted with plasma levels of some antioxidants to modulate prostate cancer risk. This observation provides additional novel evidence that inflammation and oxidative stress play a role in the etiology of this malignancy. If our findings were confirmed in large epidemiologic studies, they may offer new strategies for the prevention of prostate cancer.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
American Cancer Society (2009) Cancer facts & figures. American Cancer Society, Atlanta
Li H, Kantoff PW, Giovannucci E et al (2005) Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res 65:2498–2504
van Gils CH, Bostick RM, Stern MC, Taylor JA (2002) Differences in base excision repair capacity may modulate the effect of dietary antioxidant intake on prostate cancer risk: an example of polymorphisms in the XRCC1 gene. Cancer Epidemiol Biomarkers Prev 11:1279–1284
De Marzo AM, Marchi VL, Epstein JI, Nelson WG (1999) Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 155:1985–1992
Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR (2009) XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci USA 106:16351–16356
Dennis LK, Lynch CF, Torner JC (2002) Epidemiologic association between prostatitis and prostate cancer. Urology 60:78–83
Dennis LK, Dawson DV (2002) Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology 13:72–79
Mahmud S, Franco E, Aprikian A (2004) Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer 90:93–99
De Marzo AM, Platz EA, Sutcliffe S et al (2007) Inflammation in prostate carcinogenesis. Nat Rev Cancer 7:256–269
Goode EL, Ulrich CM, Potter JD (2002) Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev 11:1513–1530
Xu J, Zheng SL, Turner A et al (2002) Associations between hOGG1 sequence variants and prostate cancer susceptibility. Cancer Res 62:2253–2257
Stone A, Ratnasinghe LD, Emerson GL et al (2005) CYP3A43 Pro(340)Ala polymorphism and prostate cancer risk in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 14:1257–1261
Zhang J, Dhakal IB, Greene G, Lang NP, Kadlubar FF (2010) Polymorphisms in hOGG1 and XRCC1 and risk of prostate cancer: effects modified by plasma antioxidants. Urology 75:779–785
El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H (2002) Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr 76:172–179
Platz EA, De Marzo AM (2004) Epidemiology of inflammation and prostate cancer. J Urol 171(2 Pt 2):S36–S40
McCarron SL, Edwards S, Evans PR et al (2002) Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res 62:3369–3372
Bidwell J, Keen L, Gallagher G et al (2001) Cytokine gene polymorphism in human disease: on-line databases, supplement 1. Genes Immun 2:61–70
Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB (2004) The role of inflammation in the pathogenesis of prostate cancer. J Urol 172(5 Pt 2):S6–S11 discussion S11-12
Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS (2007) COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer 97:557–561
Zhang X, Miao X, Tan W et al (2005) Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology 129:565–576
Koch AE, Polverini PJ, Kunkel SL et al (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798–1801
Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ (2001) Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia 3:33–42
Inoue K, Slaton JW, Eve BY et al (2000) Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res 6:2104–2119
Veltri RW, Miller MC, Zhao G et al (1999) Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology 53:139–147
Michaud DS, Daugherty SE, Berndt SI et al (2006) Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res 66:4525–4530
Hull J, Thomson A, Kwiatkowski D (2000) Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55:1023–1027
Zhang J, Dhakal I, Stone A et al (2007) Plasma carotenoids and prostate cancer: a population-based case-control study in Arkansas. Nutr Cancer 59:46–53
Guo Y, Zhang X, Tan W et al (2007) Platelet 12-lipoxygenase Arg261Gln polymorphism: functional characterization and association with risk of esophageal squamous cell carcinoma in combination with COX-2 polymorphisms. Pharmacogenet Genomics 17:197–205
Saenz-Lopez P, Carretero R, Cozar JM et al (2008) Genetic polymorphisms of RANTES, IL1-A, MCP-1 and TNF-A genes in patients with prostate cancer. BMC Cancer 8:382
Danforth KN, Rodriguez C, Hayes RB et al (2008) TNF polymorphisms and prostate cancer risk. Prostate 68:400–407
Moore SC, Leitzmann MF, Albanes D et al (2009) Adipokine genes and prostate cancer risk. Int J Cancer 124:869–876
Pierce BL, Biggs ML, DeCambre M et al (2009) C-reactive protein, interleukin-6, and prostate cancer risk in men aged 65 years and older. Cancer Causes Control 20:1193–1203
Sun J, Hedelin M, Zheng SL et al (2004) Interleukin-6 sequence variants are not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 13:1677–1679
Stark JR, Li H, Kraft P et al (2009) Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer 124:2683–2689
Willett WC, Lenart E (1998) Reproducibility and validity of food-frequency questionnaire. In: Willett WC (ed) Nutritional epidemiology, 2nd edn. Oxford University Press, New York, NY, pp 101–147
Hughes C, Murphy A, Martin C, Sheils O, O’Leary J (2005) Molecular pathology of prostate cancer. J Clin Pathol 58:673–684
Comstock GW, Alberg AJ, Helzlsouer KJ (1993) Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin Chem 39:1075–1078
Acknowledgments
This study is supported by a fund from the Arkansas Department of Health (Dr. Zhang, PI) and a grant from the National Institute on Aging, NIH (1R01AG15722, Dr. Lang, PI). We are grateful to Angie Stone for her assistance in DNA extraction and genotyping.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Dhakal, I.B., Lang, N.P. et al. Polymorphisms in inflammatory genes, plasma antioxidants, and prostate cancer risk. Cancer Causes Control 21, 1437–1444 (2010). https://doi.org/10.1007/s10552-010-9571-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-010-9571-0