Abstract
Objective
In order to examine health inequalities in terms of incidences and case fatalities in a German health insurance population. Lung cancer, stomach cancer, intestinal carcinoma, and breast cancer were considered. Social differentiation was depicted by income and occupational position in order to examine which one is more strongly associated with incidence and case fatality.
Methods
Analyses were performed using data from a statutory health insurance (n = 170,848). Incomes were divided into quintiles, and subjects were grouped according to occupational status.
Results
For lung cancer incidence a gradient between the highest and the lowest 20% of the income distribution emerged. The relative risk of the lowest category was RR = 7.03, for occupational position the figure was RR = 6.98. For stomach cancer the relative risks were RR = 5.33 for income and RR = 7.11 for occupational position. For intestinal carcinoma only income was significantly related with incidence (RR = 4.37 for the lowest 20% of the income distribution), and for breast cancer incidence no social inequalities were found. For case fatality increased relative risks emerged for lung cancer, but only for income.
Conclusions
Income and occupational position were associated with cancer incidence with the exception of breast cancer. Apart from lung cancer, case fatalities were unrelated to measures of social differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As for many other diseases, social inequalities have been reported also for malignancies. Lung cancer incidence was reported to be higher in lower socio-economic groups [1]. This finding emerged in surveys [2] and also in registry-based studies [3], and similar results had been reported for case-fatalities [4]. Besides individual-based indicators, area-based measures were also used for classifying target populations by socio-economic status [5]. In the geographical area that was assigned the lowest category, the risk of dying due to lung cancer was about 1.6 in men between 25 and 64 years and 1.4 in men aged at least 65 years. The comparison group was made up out of individuals living in areas that were assigned the highest classification. Increased risks of contracting lung cancer had been reported in a number of studies [6–10], and variations in incidence were consistently following corresponding patterns of smoking behavior.
Gastro-intestinal cancers had less often been considered. In a Dutch study using data from 204 communities embracing 58,279 males, 162 cases of gastric cancer were identified [11]. In men with the lowest qualification the highest incidences were found. Their relative risk was 54% in individuals with the highest educational level, for stomach cancer the respective relative risk was RR = 0.37. These figures were somewhat reduced after confounders had been controlled for, but the basic findings remained unchanged. Social differences had also been found in other studies [12–14]. The evidence is, however, not consistent since in come cases incidences increased with socio-economic position [12, 15].
Breast cancer is deviating from this general pattern of health inequalities as it was reported to occur more frequently in women from higher socio-economic background [16, 17], and this holds for wealthy as well as for poor or developing countries [18]. In a study where patient records and census information were combined, breast cancer risks and social position showed a linear increase [19]. This was confirmed in an analysis embracing 97,227 women [20]. The gradients were also varying with respect to ethnic origin. Pukkala and Weiderpass analyzed all cancer cases in Finnish women with onsets between 1971 and 1995 and reported breast cancer risks to increase with social class. [21] In an age-cohort study no associations between breast cancer occurrence and socio-economic position had been reported [20].
The social distribution of breast cancer incidence differs from corresponding patterns of case fatalities. While in most studies on the relationship between breast cancer and socio-economic status positive correlations were reported this is different for death cases, although the findings are not homogeneous. Most breast cancer studies have reported higher rates in women in disadvantaged social positions [19–24], but in an Italian study higher death rates in women from higher socio-economic background were reported [25]. The socio-economic differences between incidence and deaths are remarkable and may be explained by variations in illness behavior and medical treatment, but the effects of socioeconomic position remained after these factors had been taken into account [23, 24].
In Germany, research on social inequalities in cancer is rare, and to our knowledge only a few studies are available. One of them is dealing with survival of colorectal cancer [26], but socio-economic position had been determined on the basis of patients’ residence, and the second study dealt with cancer in general [27].
Cancer registration in Germany is practiced at a regional basis [28–30], and the data are not always comparable. In contrast to other countries [23, 24], the registry data do not include information on socio-economic position, thus survival rates can only be examined on the basis of age, gender, or medical variables.
The following analyses are conducted with data from a German statutory health insurance. For Germany this is one of the few approaches to study social differences in malignant diseases on an individual basis. The dataset contains information on the frequency of treatment including dates of hospital admissions and discharges. This permits to study disease courses as far as they are documented as treatment records, and finally also the date of death is available. The disadvantage of health insurance data is the absence of information on tumor type, stage, and therapy, and the records do not include causes of death. On the other hand, individual-based data on education and on occupational position are available. Most earlier studies had been based on one indicator only, e.g., last occupation [24, 31], or education [32], and only one was based on education and occupational position [4]. It had been shown that the most frequently used indicators of socio-economic position (occupational position, education, and income) were not highly correlated, thus they must not be considered as interchangeable [33, 34]. If no association between one of these indicators and disease outcomes emerge, it may be different if another indicator is chosen. In earlier analyses it had also been shown that the relative strength of their effects were varying according to outcome [34].
In the following analyses occupational position and income will be considered together thus making it possible to compare their relative effects. As such analyses have not yet been conducted for Germany. They will contribute to closing the gap of knowledge on social inequalities of cancers.
The following questions will be dealt with:
-
Do health inequalities with respect to incidence of cancers of the lung, the stomach, the intestine, and the breast exist in this insurance population?
-
Do case fatalities of lung cancer, stomach cancer, cancer of the intestine, and breast cancer differ by social position?
-
Are health inequalities in malignant diseases more strongly associated with income or with occupational position?
Materials and methods
Study population
The following analyses are based on records of a large statutory health insurance (Allgemeine Ortskrankenkasse) covering the years 1987–1996. Actually the data were collected for accounting purposes. The dataset also includes retired and co-insured subjects, the latter mostly being wives. Due to peculiarities of the German health insurance system, lower socio-economic groups are overrepresented [35]. Retired individuals were assigned their highest occupational position attained, and jointly insured individuals were classified according to their spouses’ occupational position and income.
The study population consists of 170,764 men (56.1%) and women (43.9%) at the age of 35 up to 70 years and insured for at least 365 days. For analyses of case- fatality the age limit was extended to 75 years in order to take a five-years period [36] after disease onset into account.
Taking a population of >80,000,000 residents into account, the number of uninsured persons in Germany is low. In 1991, 232,000 were without health coverage and 105,000 in 1995. Reasons for not being covered were long-term unemployment, homelessness, and being self-employed. In the latter case income was only loosely related to the lack of health coverage [37]. In addition, the upper 10% of the income distribution are not represented in the data since the majority of them were privately insured.
The dataset is left- and right-censorized. For cancer incidence an insured person starts contributing person years at risk if exceeding the age of 35. In case of joining the insurance at a later age, periods of insurance were summed up until reaching the age of 70, until cancer was documented, or until leaving the health insurance. For case fatality persons with cancer as described above were considered from the date of documented onset to the following five years, until the date of death or until leaving the health insurance.
In Table 1 the basic description of the study population is displayed. For 57.5% of the study population income information was not available. This is due to retirement, unemployment, or to exclusions due to irregularities in the records [38].
Numbers of cancer cases were sufficient for performing regression analyses (Table 2). The survival rates of the time periods under observation are 35% (women) and 31.7% (men) for lung cancer, for stomach cancer they are 60% (women) and 55.6% (men), for intestinal carcinoma they are 70.8% (women) and 71.1% (men) and finally 70.7% for breast cancer. The survival rates in this dataset are higher than those reported from German registries [36]. This is due to cancer patients not only leaving their insurance due to death, but also by changing the insurance. Thus Table 2 may not depict all deaths due to the malignancies considered; nevertheless the results are valid, because the analyses have taken lengths of observation into account.
Occupational group membership was determined by using a three-digit classification issued by the German Labor Authority [39]. These categories were collapsed into five groups: “unskilled and semi-skilled positions,” “skilled manuals,” “skilled non-manuals,” “intermediates,” and “professionals.” Due to the small number of professionals they had to be counted together with intermediate positions, and in the regression analyses they will serve as the reference category.
Data on occupational changes are routinely transferred from employer to health insurance. In the analyses below the highest level attained was used. For a considerable proportion of the insured this information was not available (missing data, longstanding unemployment, early retirement). Despite their heterogeneity, unclassified subjects were treated as a separate group.
Income (due to employment) is also transmitted from employer to health insurance as it is the basis for calculating insurance fees. Not all subjects were insured throughout the observation period. Some had been insured before electronic data storage began (i.e., before 1987), for some individuals coverage began and ended between 1987 and 1996, others entered the insurance after 1987 and had continuous coverage. The numerical amount of wages during the observation period increased without a parallel rise of purchasing power necessarily having taken place, thus numeric monthly revenues in 1987 and 1996 are not comparable. This made it necessary to standardize individual incomes. If individuals were insured for more than one year, the amounts were converted into a one-year reference period. In order to obtain comparability, for each individual the deviation from the mean per year was calculated. The means of these deviations were computed, and served as indicator of income. In the statistical analyses, the income continuum is transformed into categories of five groups of equal size. Actually for all insured with an occupation, income information is available as this is the basis for the calculations of insurance premiums. Missing values were assigned if unusually high or unusually low payments were recorded. This concerned single payments, transitional periods with formal employment, but without payment, rehabilitation periods, etc. In case of constant insurance this did not cause classification problems, but if individuals were insured shorter, a regular income could not be calculated and missing values had to be assigned. Again subjects with missing data were classified into an own category.
Statistical analyses
The following analyses are based on Cox-regression [40, 41]. The proportional hazards model is appropriate here since it takes time (in the following analyses: age) into account. It can handle insurance periods of differing lengths, i.e., individuals leaving the population after short periods will not cause biased results. Cox regression depicts a time process, whereas it is assumed that an event (in the present case: date of diagnosis or date of death) will occur as a function of time having elapsed. For every covariate (in the present case indicators of socio-economic status and gender) it is estimated to what extent the time process is altered, i.e., whether the respective risks for defined groups decrease or increase. For incidence the time interval refers to the age at diagnosis, for deaths it is the time interval between diagnosis and death by taking age into account. All analyses were performed with STATA 10SE [42].
Results
The rank order correlation between occupational position and income is r = 0.16, thus regression effects should not be impaired by multicollinearity problems, and the two indicators should be considered as having different latent content.
In the first step, relative morbidity risks are computed. In the second step, dealing with case fatality only, patients with one of the four diseases of interest are considered with respect to differences due to socio-economic status.
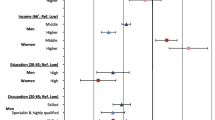
For lung cancer incidence (Table 3) monotonously increasing social gradients appear for both indicators. For the second highest 20% of the income distribution, the relative risks are not significant, while for the remaining income categories the effects are robust and range up to RR = 7.0 for the lowest 20% of the income distribution. The relative risk of unclassified subjects is falling close to the second lowest fifth.
In the analysis for occupational position, the comparison with the highest occupational category (professionals and intermediate positions combined) yields effects ranging from RR = 2.7 to 6.9, and again the relative risks are increasing monotonously with decreasing occupational position. The RR for unclassified subjects is falling between individuals holding skilled manual and unskilled/semi-skilled positions. The confidence intervals of occupational position are wide, indicating that the estimations may be affected by small case numbers.
There is a strong gender difference since in women as compared to men the age-standardized risk for a lung cancer diagnosis is only 15%.
For lung cancer deaths the results are different. For occupational position the relative risks are not significant, but this is different for income. Again compared with the highest 20% of the income distribution in the lowest 40% robust relative risks appear, suggesting considerable social inequalities additional to inequalities in incidence. The analyses also indicate that, once having contracted the disease, there is no significant gender difference for deaths.
Incidence due to stomach cancer (Table 4) could be analyzed only after having added up skilled non-manuals and the intermediate/professional positions as the rates of patients were too small in the upper categories, while for income this was not necessary. For both indicators social gradients emerged, but for occupational position they are more pronounced than for income. The relative risks for unclassified subjects are high (RR = 5.3 and 5.7). For both indicators the confidence intervals are again wide; low cell frequencies should have caused the problem.
As reported for lung cancer, there are gender differences; again in women the risk of stomach cancer is more than 50% lower than in men.
Social differentials in terms of case fatalities appeared neither for occupational position nor for income, and also no gender differences were found.
Social differentials in incidence due to cancer of the intestine (Table 5) emerged only for income. The relative risks are similar to those found for stomach cancer. For occupational position no social differences are present. Only for unclassified insured the relative risk is significant. No social differentials in case fatality are present.
For breast cancer incidence (Table 6) a significant relative risk appears only in women that could not be classified for income. For the remaining categories as well as for occupational position no social differences can be reported. No social differences for breast cancer deaths emerged.
Discussion
In the present study social gradients in incidence and case fatality of four types of malignant disease were examined. For Germany, social differentials of malignant diseases at individual level had not yet been examined, although some studies on the topic had been published [26, 27].
The most pronounced effects were found for lung cancer, and they emerged both for income and for occupational position. A substantial share of lung cancer risk is due to smoking. The social patterns of tobacco consumption were reported to be similar to lung cancer. This holds not only for Germany [43], but also for other European countries [9]. Smoking is also correlated with the main indicators of socioeconomic position, i.e., in all cases the lowest positions were associated with the highest tobacco consumption [44]. Thus, although the relative contribution of smoking to social gradients of lung cancer may vary by country, it always explains a substantial part of social inequalities of lung cancer risk [9]. To a lesser degree this applies to risk-taking behavior and to expositions to environmental hazards [45, 46]. After having considered the risks of contracting lung cancer, the second line of analysis was carried out only with patients and death as outcome. Now only income was associated with increased risks of lung cancer death. Taken together, these results point to a double social inequality in health, because disadvantaged individuals have higher risks of lung cancer, and once affected, they are also at higher risk of dying.
For stomach cancer social gradients were reported for both indicators, but no interpretable differences in case fatalities emerged. This finding is not in accordance with other European studies where excess mortalities were reported for lower socioeconomic groups [47], although group differences were sometimes small [48]. Incidences and case fatalities of stomach cancer were declining over the past decades [49]. The incidence of intestinal cancers was increasing, but case fatalities were declining slowly [30, 49]. In the analysis presented above, the social gradients were different from stomach cancer since only income was associated with increased incidence, but not with occupational position. This finding is in line with the inconsistent evidence on the associations with occupational hazards [50, 51]. Again the social gradients found in our data are consistent with some earlier studies, but this does not hold for case fatalities [5, 11]. As established for lung cancer, living conditions and lifestyle patterns are risk factors for cancer of the stomach. Besides smoking, a low consumption of fruit and vegetables as well as a preference for red meat turned out to be associated with incidence [52, 53]. Social gradients were demonstrated for all risk factors, and the unfavorable combinations were reported to occur more frequently in lower socio-economic groups. This also applies to intestinal cancers, where increased body weight, lack of physical activity, the consumption of red meat, and animal fat were suspected to increase the risk of colon cancer. For small intestine cancer the knowledge about risk factors is limited. In the literature the consumption of alcohol, smoking, and dietary factors had been reported, but the results are far from conclusive [12].
For breast cancer incidence no social gradients were found for occupational position, and for income unclassified women had elevated risks. These findings are in contrast to studies where breast cancer was found to occur more often in middle class women [19, 21, 54]. The higher relative risk in unclassified women is difficult to explain since this is a heterogeneous group consisting of single and/or unemployed women or mothers and a large subgroup that cannot be described precisely. In contrast to genetic predispositions that are unrelated to social position, a number of risk factors (older age at first pregnancy, low parity, nutrition habits) were reported to occur more frequently in higher socioeconomic groups [55], but in contrast to studies from other European countries incidences do not show social patterns.
Similar to the nearly total absence of inequalities in incidence, no social differences were found for case fatality. This differs from international studies where breast cancer deaths were related to social class or socioeconomic position [24, 56], but in a recent publication from France it was reported that social differences of breast cancer deaths disappeared after a period of decline [13]. This may indicate the absence of social gradients with respect to the utilization of health care facilities or the quality of care. However, in some studies it was found that in women with lower socio-economic background breast cancers were in a more advanced stage upon detection [57, 58]. This may be due to delay in seeking medical care in good time, which may again result in increasing risks of dying prematurely.
In the scientific discussion on health inequalities the dominating role of material conditions had been emphasized [59], but this underestimates the effects of other factors since the role of income (as the best indicator of material conditions) was not consistent. In the preceding incidence, analyses on cancers of the lung and the stomach occupational position turned out as important, but this was not the case for intestinal and breast cancer. This emphasizes that in health inequality studies more than one indicator should be included and their relative contributions have to be considered in comparison; a restriction of the scientific discussion to material conditions [60] is not appropriate. Consequently, the role of every dimension of social differentiation also has to be established for every type of cancer separately.
In the scientific literature, the role of health care systems and how medical care is delivered is discussed. This applies to cancers in general [61, 62], and for lung [63] as well as for breast cancer [64] in particular. The authors maintain that social differentials in cancer deaths may indicate barriers to appropriate treatment, and this is backed up with social differences in case fatalities [62, 63]. If the quality of health care and access to treatment would explain differences under the conditions of the German health care system, it should have effects on all types of cancers considered. With one exception this had not been the case, thus we conclude that medical treatment may not differ by socio-economic factors. A precise test of this assumption is, however, not possible since the data were actually collected for accounting purposes and do not contain information on subjective measures of health status or on the perception of symptoms.
Unequal access to treatment may be a tempting explanation for differences in survival, but the German health care system is not the only one to offer equal access. This is the case in Switzerland where social position is a prognostic factor [24], but equal access is also provided in France where social differences of survival disappeared in the 1990s [13].
Until the 1990s the average duration of stay in hospital in Germany was higher than in most neighboring countries. By that reason medical control of patients was longer, and this may have led to a social equalization of survival. However, this is an unproven argument. Under the present condition of shortening lengths of stay this may change, but the set-up of treatment centers and integrated care may again act toward better quality of treatment.
Finally, some critical issues have to be considered. The large proportion of unclassified subjects on occupational position and especially on income raises the question how to interpret the results obtained for this group. The knowledge on their socio-economic structure and information about their social environment is incomplete.
Another shortcoming of the health insurance data may affect the size of the social gradient. The data do not depict the upper 10% of the population, the same holds for subjects from the lowest positions of the social scale, e.g., the majority of individuals being on social security or homeless people. Omitting them should lead to an underestimation of social gradients with respect to incidences and case-fatalities. In the analyses above the highest occupational position obtained during the observation period had been assigned for classifying individuals for occupational position. Thus the consequences of social upward and downward mobility had not been taken into account, but this may affect disease incidence [2]. As far as the ups and downs in the study population level each other out the net effect on the results may be zero. However, at that time the data were recorded, the study region underwent economic changes which may have led to more downward than upward social mobility. Again the social gradients with respect to disease incidence may have been underestimated.
Another problem of health insurance data is the absence of information on tumor size and receptor status. Although this limits their range of application, it may not question the substantive conclusions on social differentials with respect to incidence. In earlier studies it had been shown that social inequalities in case fatalities remained also after tumor stage, treatment and patient characteristics had been taken into account [23, 24]. In a Danish study, it was found that the staging of tumors upon detection did not differ by patients’ socio-economic background [65]. However, there were differences in treatment since women from higher socio-economic background were more likely to receive lumpectomy, but how this may have affected survival is not clear.
A last uncertainty was caused by the lack of information on causes of death since only hospital diagnoses and date of death had been available. The bias resulting out of this shortcoming should be reduced by having restricted the time period after disease manifestation to five years. Eventual biases should be more effective in the age groups over 65 years, but the largest share of them is falling into the groups that could not be classified for income and occupational position.
Comparing survival rates of cancer cases of the insurance population with registry data from Germany, we may conclude that mortalities in the cancer cases from the health insurance population are likely to be due to these diseases. According to registry information [36], the mean five-year survival rates for lung cancer are 17% (women) and 9% (men), for stomach cancer the respective figures are 28% (women) and 27% (men), for intestinal carcinoma 51% (women) and 48% (men), and finally 73% for breast cancer. As the survival rates in the health insurance data are higher, a number of individuals with cancer might have left the insurance without having died and others were under observation for less than five years.
References
Hart CL, Hole DJ, Gillis CR, Smith GD, Watt GCM, Hawthorne VM (2001) Social class differences in lung cancer mortality: risk factor explanations using two Scottish cohort studies. Int J Epidemiol 30:268–274
Marshall B, Chevalier A, Garillon C, Goldberg M, Coing F (1999) Socioeconomic status, social mobility and cancer occurrence during working life: a case-control study among French electricity and gas workers. Cancer Causes Control 10:495–502
Mao Y, Hu JF, Ugnat AM, Semenciw R, Fincham S (2001) Socioeconomic status and lung cancer risk in Canada. Int J Epidemiol 30:809–817
Menvielle G, Luce D, Geoffroy-Perez B, Chastang J-F, Leclerc A (2005) Social inequalities and cancer mortality in France, 1975–1990. Cancer Causes Control 16:501–513
Singh GK, Miller BA, Hankey BF (2002) Changing area socioeconomic patterns in US cancer mortality, 1950–1998: Part II—lung and colorectal cancers. J Natl Cancer Inst 94:916–925
Braaten T, Weiderpass E, Kumle M, Lund E (2005) Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study. Cancer Epidemiol Biomarkers Prev 14:2591–2597
Brewster DH, Thomson CS, Hole DJ, Black RJ, Stroner PL, Gillis CR (2001) Relation between socioeconomic status and tumour stage in patients with breast, colorectal, ovarian, and lung cancer: results from four national, population based studies. BMJ 322:830–831
Hemminki K, Zhang H, Czene K (2003) Socioeconomic factors in cancer in Sweden. Int J Cancer 105:692–700
Mackenbach JP, Huisman M, Andersen O et al (2004) Inequalities in lung cancer mortality by the educational level in 10 European populations. Eur J Cancer 40:126–135
Faggiano F, Partanen T, Kogevinas M, Boffetta P (1997) Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ 138:65–176
van Loon AJ, Goldbohm RA, van den Brandt PA (1998) Socioeconomic status and stomach cancer incidence in men: results from The Netherlands Cohort Study. J Epidemiol Community Health 52:166–171
Pukkala E, Weiderpass E (2006) Time trends in socioeconomic differences in incidence rates of cancers of gastro-intestinal tract in Finland. BMC Gastroenterol 6:41. doi:10.1186/1471-230X-6-41
Menvielle G, Leclerc A, Chastang J-F, Luce D (2006) Social inequalities in breast cancer mortality among French women: disappearing educational disparities from 1968 to 1996. Br J Cancer 94:152–155
Brown J, Harding S, Bethune A, Rosato M (1998) Longitudinal study of socio-economic differences in the incidence of stomach, colorectal and pancreatic cancers. Popul Trends 94:35–41
Negri E, Bosetti E, LaVecchia C, Fioretti F, Conti E, Franceschi S (1999) Risk factors for adenocarcinoma of the small intestine. Int J Cancer 82:171–174
Faggiano F, Partanen T, Kogevinas M, Boffetta P (1997) Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ 138:65–176
Mielck A (2000) Soziale Ungleichheit und Gesundheit. Huber, Bern
Aziz Z, Sana S, Akram M, Saeed A (2004) Socioeconomic status and breast cancer survival in Pakistani women. J Pak Med Assoc 54:448–543
Liu L, Deapen D, Bernstein L (1998) Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States). Cancer Causes Control 9:369–380
Yost K, Perkins C, Cohen R, Morris C, Wright W (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711
Pukkula E, Weiderpass E (1999) Time trends in socio-economic differences in incidence rates of cancers of the breast and female genital organs (Finland, 1971–1995). Int J Cancer 31:56–61
Macleod U, Ross S, Twelves C, George WD, Gillis C, Watt GCM (2000) Primary and secondary care management of women with early breast cancer from affluent and deprived areas: retrospective review of hospital and general practice records. Br Med J 320:1442–1445
Kaffashian F, Godward S, Davies T, Solomon L, McCann J, Duffy SW (2003) Socioeconomic effects on breast cancer survival: proportion attributable to stage and morphology. Cancer 89:1693–6
Bouchardy C, Verkooijen HM, Fioretta G (2006) Social class is an important and independent prognostic factor of breast cancer mortality. Int J Cancer 119:1145–1151
Michelozzi P, Perucci CA, Forastiere F, Fusco D, Ancona C, Dell’Orco V (1999) Inequality in health: socioeconomic differentials in mortality in Rome, 1990–1995. J Epidemiol Community Health 53:687–693
Brenner H, Mielck A, Ziegler H (1991) The role of socioeconomic factors in the survival of patients with colorectal cancer. J Clin Epidemiol 44:807–815
Neumann G, Liedermann A (1981) Mortalität und Sozialschicht. Bundesgesundheitsblatt 24:173–181
Katalinic A, Hense H-W, Becker N (2006) Krebsregistrierung in Deutschland cancer registration in Germany. Der Onkologe 12:1084–1093
Katalinic A (2004) Epidemiologische Krebsregistrierung in Deutschland. Bestandsausfnahme und Perspektiven. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 47:422–428
Becker N, Altenburg H-P, Stegmaier C, Ziegler H (2007) Report on trends of incidence (1970–2002) of and mortality (1952–2002) from cancer in Germany. J Cancer Res Clin Oncol 133:23–35
Hall S, Holman CD, Sheiner H, Hendrie D (2004) The influence of socio-economic and locational disadvantage on survival after a diagnosis of ling and breast cancer in Western Australia. J Health Serv Res Policy 9(Suppl 2):10–16
Steenland K, Henley J, Thun M (2002) All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society Cohorts, 1959–1996. Am J Epidemiol 156:11–21
Lahelma E, Martikainen P, Laaksonen M, Aittomaki A (2004) Pathways between socioeconomic determinants of health. J Epidemiol Community Health 58:327–332
Geyer S, Hemström Ö, Peter R, Vågerö D (2006) Education, income and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against an unquestioned practice. J Epidemiol Community Health 60:804–810
Geyer S, Peter R (1999) Occupational status and all-cause mortality: a study with health insurance data from Nordrhein-Westfalen, Germany. Eur J Public Health 9:114–118
Arbeitsgemeinschaft Bevölkerungsbezogener Krebsregister in Deutschland (2002) Krebs in Deutschland. Häufigkeiten und Trends (Cancer in Germany. Frequencies and trends). Saarbrücken
Greß S, Walendzik A, Wasem J (2005) Nichtversicherte Personen im Krankenversicherungssystem der Bundesrepublik Deutschland—Bestandaufnahme und Lösungsmöglichkeiten (Uninsured persons in the health care system of the Federal Republic of Germany- Stocktaking and possible solutions). Faculty of the Economic Sciences, University of Duisburg- Essen, Essen
Geyer S, Peter R (2000) Income, social position, qualification and health inequalities-competing risks? J Epidemiol Community Health 54:299–305
Bundesanstalt für Arbeit (1992) Schlüsselverzeichnis für die Angaben zur Tätigkeit in den Versicherungsnachweisen (Code manual for occupational information in insurance certificates). Bundesanstalt für Arbeit, Nürnberg
Cox DR, Oakes D (1984) Analysis of survival data. Chapman & Hall, London
Collett D (1994) Modelling survival data in medical research. Chapman & Hall, London
Stata Corp. (2007) Stata statistical software: release 10. College Station, TX
Helmert U, Borgers D, Bammann K (2001) Soziale Determinanten des Rauchverhaltens in Deutschland: Ergebnisse des Mikrozensus 1995. Sozial- und Präventivmedizin 46:172–181
Laaksonen M, Rahkonen O, Karvonen S, Lahelma E (2005) Socioeconomic status and smoking: analysing inequalities with multiple indicators. Eur J Public Health 15:262–269
Evans GW, Kantrowitz E (2002) Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health 23:303–331
Ernster VL (1996) Female lung cancer. Annu Rev Public Health 17:97–114
Stephens MR, Blackshaw GR, Wyn GL et al (2005) Influence of socio-economic deprivation on outcomes for patients diagnosed with gastric cancer. Scand J Gastroenterol 40:1351–1357
Hemminki K, Zhang H, Czene K (2003) Socioeconomic factors in cancer in Sweden. Int J Cancer 105:692–700
Becker N, Wahrendorf J, Holzmeier S (2002) Krebsatlas der Bundesrepublik Deutschland 1981–1990. Atlas of Cancer Mortality in the Federal Republic of Germany. Springer, Heidelberg
Chow WH, Malker HS, Hsing AW et al (1994) Occupational risks for colon cancer in Sweden. J Occup Med 36:647–651
DeRoos AJ, Ray RM, Gao DL et al (2005) Colorectal cancer incidence among female textile workers in Shanghai, China: a case-cohort analysis of occupational exposures. Cancer Causes Control 16:1177–1188
Gonzalez CA, Jakszyn P, Pera G et al (2006) Meat intake and risk of stomach and esophagal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC-EURGAST). J Natl Cancer Inst 98:345–354
Kono H, Hirohata T (1996) Nutrition and stomach cancer. Cancer Causes Control 7:41–55
Krieger N, Quesenberry C, Peng T, et al (1999) Social class, race/ ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, Black, Hispanic and White residents of the San Francisco Bay area 1988–92 (United States). Cancer Causes Control 10:525–537
Kogevinas M, Porta M (1997) Socioeconomic differences in cancer survival: a review of the evidence. IARC Sci Publ 138:177–206
Oksbjerg Dalton S, Ross L, Düring M, et al (2007) Influence of socioeconomic factors on survival after breast cancer—a nationwide cohort study of women diagnosed with breast cancer in Denmark 1983–1999. Int J Cancer. doi:10.1002/ijc.22979
Baquet CR, Commiskey P (2000) Socioeconomic factors and breast carcinoma in multicultural women. Cancer 88(Suppl 5):1256–1264
Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS (1998) Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. J Am Med Assoc 279:1801–1807
Wilkinson RG (2005) The impact of inequality. How to make sick societies healthier. Routledge, London
Subramanian SV, Belli P, Kawachi I (2002) The macroeconomic determinants of health. Annu Rev Public Health 23:287–302
Teppo L, Dickman PW, Hakulinen T, et al (1999) Cancer patient survival—patterns, comparisons, trends—a population-based cancer registry study in Finland. Acta Oncol 38:283–294
Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM (2000) An international comparison of cancer survival: relatively poor areas of Toronto, Ontario and three US metropolitan areas. J Public Health Med 22:343–348
Gorey KM, Holowaty EJ, Laukkanen E, Fehringer G, Richter NL (1998) Association between socioeconomic status and cancer incidence in Toronto, Ontario: possible confounding of cancer mortality by incidence and survival. Cancer Prevention Control 2:236–241
Lee-Feldstein A, Feldstein PJ, Buchmueller T, Katterhagen G (2001) Breast cancer outcomes among older women—HMO, fee-for- service, and delivery system comparisons. J Gen Intern Med 16:189–199
Norredam M, Groenvold M, Petersen JH, Kresnik A (1998) Effect of social class on tumour size at diagnosis and surgical treatment in Danish women with breast cancer. Soc Sci Med 47:1659–1663
Acknowledgments
Work with this dataset was made possible by the Allgemeine Ortskrankenkasse Mettmann, especially by Klaus W. Weber and Reiner Rosenthal, who opened opportunities for using the health insurance data for scientific analyses. The help by Richard Peter, Andrea Jung, Irene Jung, Christof Lebek, and Margret Stolz is particularly acknowledged for their advice and for discussions while preparing the material used for this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geyer, S. Social inequalities in the incidence and case fatality of cancers of the lung, the stomach, the bowels, and the breast. Cancer Causes Control 19, 965–974 (2008). https://doi.org/10.1007/s10552-008-9162-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-008-9162-5