Abstract
Body size relatively early in life may influence men’s later rate of prostate cancer. We searched for published, English-language studies of the association between prostate cancer incidence or mortality and body size between ages 5 and 29 years. We summarized analyses of childhood body size, early-adult waist circumference, and early-adult body mass index (BMI). Most study designs were case–control or retrospective cohort studies, in which body size was self-reported and recalled. The few studies of childhood body size and early-adult waist circumference indicated null or weak associations. The results from studies of early-adult BMI were heterogeneous (p = 0.04) and showed evidence of funnel plot asymmetry. The random-effects rate ratio (RR) was 1.06 (95% confidence interval [CI]: 0.99, 1.14) per five-unit increase in BMI. Studies using measured (as opposed to self-recalled) height and weight (n = 3) tended to produce stronger associations: fixed-effects summary RR = 1.22 (1.06, 1.39). The same was true for studies that did not adjust for later-life BMI (n = 13): fixed-effects RR = 1.13 (1.06, 1.21). Examining only analyses of advanced or high-grade prostate cancers, results were heterogeneous (p < 0.01). The random-effects summary RR per five units of BMI was 1.01 (95% CI: 0.89, 1.15). In all the reviewed studies, the vast majority of men were of normal weight in childhood and early adulthood. Few studies presented data describing the association between prostate cancer and obesity (e.g., early-adult BMI ≥ 30). The exact relationships between early-life body size and prostate cancer remain unclear but appear to be weak.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body size may be important in the development of prostate cancer. In a recent review, however Freedland and Platz, noted that most prostate cancer research has focused on body size relatively late in life and thus “may have missed the window when excess body mass index and its sequeal would have affected prostate cancer risk” [1]. In evaluating prostate cancer risk in relation to body size relatively late in life, the authors were able to consult a systematic review of 31 cohort studies and 25 case–control studies [2]. For body size earlier in life, the authors cited only four studies, which gave the impression of having “demonstrated mixed results” [1].
Without a systematic review of the entire published literature on prostate cancer and body size relatively early in life, impressions must be developed on the unsatisfactory basis of examining an incomplete selection of the literature one study at a time. Therefore, the main goal of the present systematic review is to fill this gap in the prostate cancer literature. We provide as complete a catalog as possible of the published English-language epidemiologic studies that have investigated the association between prostate cancer and body size early in life. Where possible, we place the studies on a common quantitative footing; examine heterogeneity to assess the informal impression of a conflicting literature; investigate funnel plot asymmetry for signs of publication bias or other distorting influences; and provide summary estimates of the associations when appropriate.
Materials and methods
Literature search
We sought published, English-language research, where (1) the exposure was body size (excluding weight or adult height) between ages 5 and 29 years and where (2) the outcome was prostate cancer incidence or mortality. We chose not to examine weight because it is a relatively non-specific measure that does not differentiate among the effects of height, fat mass, and lean body mass. We chose not to examine adult height because it was recently treated by another reviewer [2].
In order to identify relevant studies published before 12 November 2007, we searched using four methods: literature searches in (1) PubMed MEDLINE and (2) ISI Web of Science Citation Index Expanded; (3) manual searches of the bibliographies of review articles and recovered articles; and (4) manual searches of all the articles that had cited recovered articles (ISI Web of Science Cited Reference Search). Our PubMed and Web of Science keyword searches were designed in consultation with two reference librarians at the University of North Carolina Health Sciences Library. The ISI Web of Science search terms were “prostate cancer” together with (1) “young, adolescent*, infant*, child*, college, early life, or puberty” and (2) “body size, obesity, BMI, body mass index, or weight.” The PubMed search terms were “human,” “male,” and “prostatic neoplasm” together with (1) “epidemiology” or “etiology” and (2) one of the following: “body weight,” “body mass index,” “overweight,” “obesity,” or “body size.” (We excluded PubMed’s age-specific keywords, “child*” and “adolescent*,” from our final search because restricting by them excluded relevant articles.) We directly examined all papers whose abstracts indicated the possibility that pertinent information was collected (e.g., study administered questionnaires about participants’ health histories). Two publications reported results from the same analysis [3, 4]; we excluded the later, less-detailed report [3].
Meta-analysis: data extraction and author contacts
Where studies examined quantitatively measured body size, we attempted to perform meta-analysis. When the published reports did not include information necessary for meta-analysis, we contacted the study authors. None of the studies included all of the information we desired for meta-analysis. Therefore, we contacted authors of all studies except the two that did not include analyses of quantitatively measured body size [5, 6].
Of the 20 sets of authors whom we attempted to contact [3, 7–25], we eventually reached 17 [3, 7–9, 11–23]; 15 were able to supply some or all of the requested information [7, 9, 11–23] (see Acknowledgments). In the end, the meta-analysis included results from 16 publications: those 15 publications whose authors supplied information and one publication whose authors we could not contact [10]. All 16 publications researched prostate cancer’s relationship with BMI. Two of those publications also analyzed prostate cancer’s relationship with waist circumference [19, 21]; another included an analysis with estimated lean body mass [23].
About 8 of the 16 studies reported results for advanced or high-grade prostate cancers [11, 12, 15, 17, 19, 21–23]. In seven cases, we had adequate information to analyze these results together in a sub-analysis restricted to advanced prostate cancer cases [11, 12, 15, 17, 19, 21, 22]. These seven studies defined advanced cancer using various criteria: (1) tumors that, at diagnosis, had metastasized or extended beyond the prostatic capsule (n = 4) [11, 12, 21, 22] or cancers with a TNM stage of T3–4, M0 or T0−4, M1 [26] (n = 1) [15]; (2) tumors with a Gleason score ≥8 [27] (n = 1) [19]; or (3) prostate cancer listed on the death certificate as a cause of death (n = 1) [17].
Meta-analysis: statistical analysis
For meta-analyses of quantitative body size, we first placed the different studies’ effect measures on the same footing by calculating a common measure of association: the natural log of the continuous rate ratio (RR) for a given increase in the body size measure. For BMI, we chose a five-unit increase, the same as that used by MacInnis and English in their meta-analysis of prostate cancer in relation to later-life BMI [2].
Throughout this article, the term “rate ratio” (RR) is used to describe incidence rate ratios estimated directly in cohort studies, by the risk ratio in cohort studies, or by the exposure odds ratio in case–control studies. We required that the studies’ RR estimates be adjusted for age or birth cohort or arise from age-matched study populations. In the one case where multiple age-adjusted estimates were available [21], we preferentially selected the estimate that was not adjusted for body size later in life (adjustment for this potential mediator of early-adult BMI’s association with prostate cancer could bias the estimates [28–32]) but that was otherwise adjusted for the greatest number of potential confounders.
In seven publications, continuous RR estimates were neither published nor provided by the study’s authors [9, 12, 14, 16, 19, 22, 23]. Six of these studies reported RR estimates in categories of BMI, waist circumference, or estimated lean body mass [12, 14, 16, 19, 22, 23]. In these cases, we used Greenland and Longnecker’s covariance-corrected generalized least-squares trend (GLST) estimation method to estimate one continuous RR for each analysis [33]. To each BMI or waist circumference category, we assigned values equal to that category’s median value for either the whole population (for cohort studies [12, 16]) or for the control group (in case–control studies [14]). (In one case–control study, which analyzed BMI and waist circumference in the same population, it was unclear whether the medians were for the control group or for cases and controls combined [19].) We implemented the GLST technique using user-written SAS code and replicated the results using Stata’s glst command [34]. The other analysis in which a continuous RR estimate was not available was a cohort study [9] about which we obtained the following for each quintile of the BMI distribution: (a) the absolute rates of prostate cancer, (b) the median BMI, and (c) the estimated number of cases and non-cases. With this information, we estimated the log-RR trend using a generalized linear model with the binomial outcome distribution and the natural-log link.
Once continuous RR trends were determined for all studies, we examined funnel plot asymmetry in three ways [35]: (1) Begg and Mazumdar’s rank order correlation test (α = 0.20) [36]; (2) the regression test of Egger et al. (α = 0.20) [37]; and (3) Duval and Tweedie’s trim and fill imputation method [38, 39]. In addition, overall homogeneity was tested using Cochran’s Q statistic (α = 0.20). When study RRs showed evidence of heterogeneity, we did not report fixed-effects summary estimates. In order to explore whether particular study characteristics were associated with average log RRs among studies [40, 41], we performed stratified analyses and REML (restricted maximum likelihood) random-effects meta-regressions [42]. Each meta-regression estimated a ratio of the average random-effects RRs from studies grouped by particular study characteristics.
The study characteristics examined under meta-regression and stratified analyses were as follows: year of publication; study design (cohort or case–control); outcome (incident cancer or fatal cancer); study location (North America; Asia; or Europe/Australia); percent (≤1.25% or >1.25%) of population who were obese (BMI ≥ 30 or waist circumference ≥102 cm); pervasiveness of prostate-specific antigen (PSA) screening when cases were diagnosed (more common in U.S. after 1988, Australia after 1993, Canada after 1990; less common in all other cases); whether the RR trend estimate was reported by study authors or estimated by us; body size assessment method (measured by study staff or recalled by study participants); age for which body size was assessed (“usual” size during their 20s; most college-aged [18 to 22-years old]; or most 25 to 29-years old); and covariate adjustment. To explore the effect of covariate adjustment, we performed meta-regression and stratified analysis for family history of prostate cancer; later-life BMI; and any indicator of socioeconomic position. Due to of the small number of studies, we were unable to conduct multiple meta-regression analyses and could only examine the study characteristics one at a time.
Results
We identified and analyzed results from 18 studies, some of which included analyses of multiple body size measures. In ten studies, the source population was the general population [7, 9–11, 13, 14, 18–21]; two populations were cohorts of university students [16, 17]; three populations consisted of respondents to mailed questionnaires [12, 15, 22]; and two were hospital-based [5, 6]. The source population was unclear in another study [23]. Half of the studies used case–control designs; six used retrospective cohort designs; and three used prospective cohort designs. Almost all populations were more than 90% Caucasian. Only four studies included more than a negligible proportion of non-Caucasians: two studied populations of Asian descent [9, 14], one study population was 20% African-American [21]; another population was 8% African-American [23].
Of the 18 studies, 16 analyzed BMI in early adulthood (18–29 years) [7, 9–23] (Table 1). Eight studies presented analyses of other body size measures: Stunkard figures for pre-adolescence [12, 14, 21]; comparison to peers during “puberty” or the early teen years [5, 6, 10]; Stunkard figures for early-adulthood [12, 14, 21]; waist circumference in the 20s [19, 21]; or estimated lean body mass [23].
Pre-adolescence and adolescence—qualitative body size measures
Pre-adolescent size was examined in three studies [12, 14, 21]; peri-puberty was examined in three other studies [5, 6, 10] (Table 2). Two of the peri-puberty studies used hospital-based controls [5, 6]. Measures of pre-adolescent and adolescent body size could not be placed onto a common quantitative footing to support meta-analytic calculations.
Two of the three studies examining pre-adolescence reported that the rate of advanced prostate cancer was somewhat lower among the heaviest pre-adolescent boys (Table 2). Studies examining peri-puberty did not indicate any associations between prostate cancer and self-reported “size” or “weight” in comparison to peers.
Early adulthood—qualitative body size measures
The age range of the 20s was examined in the same three studies that investigated body size in pre-adolescence [12, 14, 21] (Table 2). The studies’ results for the 20s were similar to those for pre-adolescence: the two North American studies appeared to show decreased rates for the heaviest young men.
Early adulthood—body mass index: meta-analysis
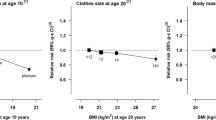
We performed a meta-analysis of the associations between prostate cancer and BMI in early adulthood (Fig. 1). Approximately 13,193 cases of prostate cancer were included in the main analysis. In the analysis of all 16 studies, there was evidence of funnel plot asymmetry (Begg–Mazumdar p = 0.24; Egger p = 0.02) and also of heterogeneity (p = 0.04). The trim and fill method imputed six hypothetically missing results, reducing the random-effects summary RR estimate from 1.06 (95% CI: 0.99, 1.14) to 1.01 (95% CI: 0.94, 1.08).
Stratifying by study characteristics, certain subgroups of studies appeared to be homogenous enough to estimate fixed-effects summary RRs (Table 3). This subgroup included studies from Europe and Australia, which overlapped a subgroup we designated as “pre-PSA” screening and another of studies in which obesity prevalence was less than 1.25%. Other homogenous subgroups were studies that did not include later-life BMI was not a covariate and those in which height and weight were measured. In all the subgroups mentioned above, the fixed-effects summary RRs were the same as the random-effects RRs in Table 3. These summary RRs ranged from 1.11 to 1.22 per five units of BMI.
In the meta-regression analyses, several study characteristics appeared to be associated with the studies’ RRs (Table 3). Studies with greater proportions of obese males tended to have lower RRs. Three study characteristics perhaps associated with a predominance of localized cancers (publication year, post-PSA era, and North-American location) were suggestively associated with lower study RRs. In addition, the three analyses that adjusted for later-life BMI produced slight inverse associations as opposed to the other 13 studies’ positive average associations. The analyses in which height and weight were measured (n = 3) had stronger positive associations than those in which the measures were recalled by study participants.
We examined BMI’s association with advanced prostate cancer in two ways. First we compared studies in which the prostate cancers were primarily diagnosed before PSA screening was widespread to those diagnosed after screening became more common. “Pre-PSA” studies tended to yield greater RRs. Second, we calculated a summary RR for BMI analyses limited to advanced-stage, high-grade, and fatal cancer cases (see Table 4). There was evidence of heterogeneity (p < 0.01) but not funnel plot asymmetry. The average of the studies’ RRs was very close to the null: random-effects RR = 1.01 (95% CI: 0.89, 1.14). The evidence of heterogeneity was attenuated (p = 0.3) when we excluded the two analyses that adjusted for later-in-life BMI [12, 43]. The fixed-effects summary RR based on the remaining five analyses was 1.07 (95% CI: 1.04, 1.11) per five-unit BMI change.
Early adulthood—waist circumference
Only two analyses examined waist circumference [19, 21]. Therefore, we did not perform a meta-analysis of these results. In the Giles et al. study [19], the estimated trends per five inches of age 21 waist were OR = 1.04 (95% CI: 0.98, 1.10) among all prostate cancer cases and OR = 1.07 (95% CI: 0.97, 1.17) among only high-grade cases. When Robinson et al. [21] examined the relationship between usual trouser waist size at age 20–29 years and advanced prostate cancer, they found a stronger relationship: OR = 1.28 (95% CI: 1.03, 1.59). In categorical analysis, however, those reporting the largest waist circumference (≥38 inches) appeared to have decreased incidence of cancer: OR = 0.73 (95% CI: 0.40, 1.35) versus those reporting waist of 32–37 inches.
Early adulthood—lean body mass
Liu et al. analyzed the relationship between prostate cancer diagnosis and an estimate of lean body mass [23]. The analysis suggested an inverse association (OR = 0.84 [0.69, 1.02]). However, the equation used to estimate lean body mass at age 20 reduces to a function of height and weight. Therefore, the lean body mass estimate may add little information above that study’s analysis of BMI, which was included in the meta-analysis of early-adult BMI.
Discussion
The studies reviewed here give the impression of null or weak associations between early-life body size and prostate cancer diagnosis. Although there exists particular interest in body size during puberty, only three studies examined this issue. The results of these studies are not definitive: there were limitations in the studies’ designs (two used hospital-based controls) and adiposity was measured with imprecision. The relationship between prostate cancer and early-adult waist circumference also remains unclear. The results of the two studies examining waist circumference and advanced or high-grade prostate cancer were compatible with a null or slightly increased incidence of advanced or high-grade prostate cancer with larger waist circumference. However, two studies is too few to draw conclusions.
The majority of the reviewed studies included analyses of early-adult BMI. Assuming a linear association between BMI and prostate cancer, these studies indicated a null or weak positive relationship. Similarly, in regard to advanced or high-grade prostate cancer, if the association is linear, it is likely null or very weakly positive. There are several limitations to the literature on advanced and high-grade prostate cancer. First is the potential for publication bias: half of the reviewed studies did not report results stratified by outcome severity. Second is the potential for selection bias: among the analyses that stratified by prostate cancer severity, 20% of cancer cases were typically unstaged and therefore excluded from the analyses. Excluding cases with this missing data could induce selection bias if having an unstaged cancer were associated with early-adult BMI. Finally, several different methods were used to classify cancer severity.
There is great interest in whether, in early life, obesity (as opposed to variation in BMI within the non-obese range, which is more closely associated with non-fat mass in men than with fat mass) is associated with prostate cancer incidence or mortality [1, 9, 12, 44]. Recent investigations of BMI in mid-life suggest that the rate of localized prostate cancer increases with higher BMI in the “normal” range (BMI < 25.0) but decreases with higher BMI in the “obese” range (BMI ≥ 30) [1]. The relationship between early-life BMI and prostate cancer could be similarly non-linear.
Unfortunately, the literature on prostate cancer and early-life body size includes very few study participants who were obese when young (see Table 1, column “percent obese”). The small number of obese study participants reflects the historically low prevalence of obesity among boys and young men, ranging from about 3 to 9% in national U.S. surveys from the 1960s and 1970s [45, 46]. Obesity prevalence did not rise dramatically in the U.S. until the 1980s [45–47]. Men who came of age during this period will not reach their 60s (and thus be at substantial risk of prostate cancer diagnosis) until the 2020s. Until then, attempts to describe the epidemiology of early-life obesity’s association with prostate cancer will be hampered by small sample sizes. Progress in understanding this relationship may depend on the analysis of pooled data or the exploitation of historical cohorts enriched with obese children and young men.
Among the studies reviewed here, only three presented or discussed BMI results that were in any way related to obesity [10, 12, 21]. Two studies presented categorical results for relatively high BMIs (i.e., median of BMI category >27) and therefore were likely to include a substantial proportion of obese men in that category [12, 21]. Both these studies observed decreased rates of advanced cancer (i.e., extraprostatic or metastatic) compared to lower referent BMI categories. In addition, the only other study to comment on this issue reported that, “for advanced cancers there were suggestions that ... high BMI [was associated] with a decreased risk (data not shown)” [10].
Thus, the available evidence is extremely limited but suggests that early-adult obesity may be inversely associated with diagnosis of advanced prostate cancer but not necessarily with diagnosis of localized prostate cancer. If true, this association would be in contrast with the literature examining BMI after the fifth decade of life, where obesity appears inversely associated with localized and low-grade disease but not necessarily advanced and high-grade disease [1, 48]. One possible explanation for the stronger inverse association with early-life versus later-life obesity may be that early-life obesity is more strongly associated with later diabetes incidence, which appears inversely associated with high-grade prostate cancer, independent of later-life obesity [48].
Because there was evidence of heterogeneity among the study RRs, we refrained from reporting one overall fixed-effects RR. However, excluding the three studies that adjusted for later-life BMI attenuated the heterogeneity: the fixed-effects RR for the remaining 13 studies was 1.13 (1.06, 1.21). Excluding the three studies that adjusted for later-life BMI is appropriate because holding later-life BMI constant could bias those studies’ early-adult BMI coefficients [28–32]. In particular, controlling for later-life BMI artificially constrains all men to have the same BMI in later-life. Imagine selecting a study population based on the fact that all men have the same BMI (e.g., BMI = 29) at age 50. In order to have arrived at the same size at age 50, the men who had low BMI at age 20 (e.g., BMI = 19.5) had to have gained much more weight between ages 20 and 50 than those with higher BMIs at age 20 (e.g., BMI = 25). Thus, the coefficient for early-adult BMI becomes a proxy for less weight gain: low early-adult BMI represents large weight gain; high early-adult BMI represents smaller weight gain.
Another source of heterogeneity among studies was the use of recalled body size instead of measured height and weight. Stevens et al. reported that the accuracy of BMI calculated from recalled weight decreased over 28 years [49]. Because most men in these studies were recalling body size from 40 years earlier, use of recalled weight in most studies may have biased estimates towards the null.
We chose not to rank the studies using quality scores. No one set of studies were free from potential bias. For instance, two studies used cohort designs, sampled from a general population, had good response rates, and had good case ascertainment [9, 11]. However, in both cases, height and weight were recalled. In contrast, three other cohort studies used measured height and weight but tended to have lower follow-up rates, less generalizable source populations in two cases [16, 17], and arguably poorer case ascertainment in two cases [16, 17]. Without knowing what degree of bias is introduced by each aspect of study design, we considered it inadvisable to assess studies based on quality assessment scales [50, 51].
This systematic review provides a summary of the published observational literature, with all its limitations. Associations between body size and less effective screening and treatment could induce biases in this literature [1, 43, 52, 53]. In addition, methodologic flaws were present to some extent in all studies. The study populations were mostly of European descent. Conspicuously under-represented were African-Americans, a group who suffer a high burden of prostate cancer. An important additional limitation of the meta-analysis is the assumption of a linear relationship between prostate cancer rates and BMI. If the association is actually non-linear (i.e., if it plateaus at high BMI or even begins decreasing), then the summary trends estimated are invalid. Investigation of this hypothesis may require a pooling project, where raw data are gathered from all studies and re-analyzed using non-linear parametric models or using splines.
Funnel plot asymmetry suggested an upward bias in the literature such that null and inverse associations may not have been as likely to be published as results more in line with expectations of a positive association. Methodologic flaws were present to some extent in all studies and may have biased associations towards the null. With two competing sources of bias likely—away-from-the-null publication bias and toward-the-null study-specific methodologic biases—a stronger association may be obscured in the literature. However we believe that whatever effect there might be between rate of prostate cancer and early-life body size is probably weak.
References
Freedland SJ, Platz EA (2007) Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev 29:88–97
MacInnis RJ, English DR (2006) Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control 17(8):989–1003
Whittemore AS, Paffenbarger RS Jr, Anderson K, Lee JE (1984) Early precursors of urogenital cancers in former college men. J Urol 132(6):1256–1261
Whittemore AS, Paffenbarger RS Jr, Anderson K, Lee JE (1985) Early precursors of site-specific cancers in college men and women. J Natl Cancer Inst 74(1):43–51
Ilic M, Vlajinac H, Marinkovic J (1996) Case–control study of risk factors for prostate cancer. Br J Cancer 74(10):1682–1686
Dal Maso L, Zucchetto A, La Vecchia C, Montella M, Conti E, Canzonieri V et al (2004) Prostate cancer and body size at different ages: an Italian multicentre case–control study. Brit J Cancer 90(11):2176–2180
Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG (2004) Case–control study of anthropometric measures and prostate cancer risk. Int J Cancer 110(2):278–283
Greenwald P, Damon A, Kirmss V, Polan AK (1974) Physical and demographic features of men before developing cancer of the prostate. J Natl Cancer Inst 53(2):341–346
Nomura A, Heilbrun LK, Stemmermann GN (1985) Body mass index as a predictor of cancer in men. J Natl Cancer Inst 74(2):319–323
Andersson SO, Baron J, Wolk A, Lindgren C, Bergstrom R, Adami HO (1995) Early life risk factors for prostate cancer: a population-based case–control study in Sweden. Cancer Epidemiol Biomarkers Prev 4(3):187–192
Cerhan JR, Torner JC, Lynch CF, Rubenstein LM, Lemke JH, Cohen MB et al (1997) Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8(2):229–238
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1997) Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 6(8):557–563
Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT (1997) A case–control study of diet and prostate cancer. Br J Cancer 76(5):678–687
Hsing AW, Deng J, Sesterhenn IA, Mostofi FK, Stanczyk FZ, Benichou J et al (2000) Body size and prostate cancer: a population-based case–control study in China. Cancer Epidemiol Biomarkers Prev 9(12):1335–1341
Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA (2000) Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol 151(6):541–549
Lee IM, Sesso HD, Paffenbarger RS Jr (2001) A prospective cohort study of physical activity and body size in relation to prostate cancer risk (United States). Cancer Causes Control 12(2):187–193
Okasha M, McCarron P, McEwen J, Smith GD (2002) Body mass index in young adulthood and cancer mortality: a retrospective cohort study. J Epidemiol Community Health 56(10):780–784
Engeland A, Tretli S, Bjorge T (2003) Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer 89(7):1237–1242
Giles GG, Severi G, English DR, McCredie MR, MacInnis R, Boyle P et al (2003) Early growth, adult body size and prostate cancer risk. Int J Cancer 103(2):241–245
Jonsson F, Wolk A, Pedersen NL, Lichtenstein P, Terry P, Ahlbom A et al (2003) Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer 106(4):594–599
Robinson WR, Stevens J, Gammon MD, John EM (2005) Obesity before age 30 years and risk of advanced prostate cancer. Am J Epidemiol 161(12):1107–1114
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T et al (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4):675–684
Liu X, Rybicki BA, Casey G, Witte JS (2005) Relationship between body size and prostate cancer in a sibling based case–control study. J Urol 174(6):2169–2173
Littman AJ, White E, Kristal AR (2007) Anthropometrics and prostate cancer risk. Am J Epidemiol 165(11):1271–1279
Cox B, Sneyd MJ, Paul C, Skegg DCG (2006) Risk factors for prostate cancer: A national case–control study. Int J Cancer 119(7):1690–1694
Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D et al (2002) AJCC cancer staging manual, 6th edn. Springer
Gleason D (1977) Histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M (ed) Urologic pathology: the prostate. Lea and Febiger, Philadelphia, pp 171–198
Weinberg CR (1993) Toward a clearer definition of confounding. Am J Epidemiol 137(1):1–8
Rothman KJ, Greenland S (1998) Modern epidemiology, 2nd edn. Lippincott-Raven, Philadelphia, 125 pp
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research: principles, quantitative methods. Lifetime Learning Publications, Belmont, CA, 257 pp
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48
Breslow NE, Day NE (1980) Statistical methods in cancer research, vol I - the analysis of case–control studies. IARC Sci Publ. (32), 107 pp
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6(1):40–57
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53(11):1119–1129
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 315(7109):629–634
Duval S, Tweedie R (2000) A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 95(449):89–98
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2):455–463
Thompson SG, Higgins JP (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21(11):1559–1573
Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18(20):2693–2708
Sharp SJ (1998) Meta-analysis regression. Stata Tech Bull 42:16–22
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T et al (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4):675–684
Freedland SJ, Aronson WJ (2005) Obesity and prostate cancer. Urology 65(3):433–439
Troiano RP, Flegal KM (1998) Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics 101(3 Pt 2):497–504
Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL (1998) Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 22(1):39–47
Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL (1995) Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med 149(10):1085–1091
Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW et al (2006) Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15(10):1977–1983
Stevens J, Keil JE, Waid LR, Gazes PC (1990) Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol 132(6):1156–1163
Juni P, Witschi A, Bloch R, Egger M (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282(11):1054–1060
Greenland S, O’Rourke K (2001) On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2(4):463–471
Freedland SJ, Giovannucci E, Platz EA (2006) Are findings from studies of obesity and prostate cancer really in conflict? Cancer Causes Control 17(1):5–9
Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV et al (2007) Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 16(1):63–69
Acknowledgments
We thank the following study authors and staff members for their generous help and correspondence: James R. Cerhan; Brian Cox; Anders Engeland; Graham G. Giles; Ed Giovannucci; Christine M. Friedenreich; Peter Greenwald; Ann W. Hsing; M. Ilic, H. Vlajinac, and Sandra Grujicic; F. Jonsson and Maria Feychting; T. J. Key and Paul N. Appleby; I-Min Lee; Xin Liu and John Witte; Abe Nomura and Grant N. Stemmerman; Mona (Okasha) Jeffreys; Agnes G. Schuurman and P. A. van den Brandt; Alice S. Whittemore; and Margaret E. Wright. The following people assisted us in finding study authors or study information: George Rhoads, Sven-Olof Andersson, Pieter C. Dagnelie, Leo Schouten, and Marjan van den Akker. We extend special appreciation to Sean Cody at the NIH/NHLBI for providing information from the A. N. et al. study. Cande Ananth wrote the SAS code used for generalized least-squares trend (GLST) estimation. Financial support: Dr. Poole received partial support for this work from NIEHS grant P30ES10126. Ms. Robinson received support from the Thomas S. and Caroline H. Royster, Jr. Fellowship (University of North Carolina, Chapel Hill); the Center for Human Science; and grant T32-CA09330 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, W.R., Poole, C. & Godley, P.A. Systematic review of prostate cancer’s association with body size in childhood and young adulthood. Cancer Causes Control 19, 793–803 (2008). https://doi.org/10.1007/s10552-008-9142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-008-9142-9