Abstract
Objective
To conduct a systematic review and meta-analyses of published studies examining the association between myeloid leukemias (ML) and occupational pesticide exposure.
Methods
Studies were identified from a MEDLINE search through 31 May 2006 and from the reference lists of identified publications. Studies were summarized and evaluated for publication bias. Relative risk estimates for ML were extracted from 17 cohort and 16 case–control studies published between 1979 and 2005. Fixed- or random-effect meta-analysis models were used depending on the presence of heterogeneity between studies. Separate analyses were conducted after stratification for study design, occupational group, ML subtype or gender.
Results
The overall meta-rate ratio estimate (meta-RR) for the cohort studies was 1.21 (95% confidence interval [CI] 0.99–1.48). Substantial heterogeneity existed among cohort studies (p = 1.064 × 10−5), mainly reflecting the varying occupational categories examined. The meta-RR was 6.32 (95% CI: 1.90–21.01) for manufacturing workers and 2.14 (95% CI: 1.39–3.31) for pesticide applicators. After stratification of cohort studies by specific ML subtype, an increased risk of acute myeloid leukemia (AML) was found (meta-RR: 1.55; 95% CI: 1.02–2.34). No significant heterogeneity was detected among case–control studies and an increased risk of chronic myeloid leukemia (CML) was found among men (meta-RR: 1.39; 95% CI: 1.03–1.88) and farmers or agricultural workers (meta-RR: 1.38; 95% CI: 1.06–1.79).
Conclusion
The strongest evidence of an increased risk of ML comes from manufacturing workers and pesticide applicators. Further studies will be needed to correlate reliable exposure data within these specific occupational groups with well-defined subtypes of leukemia to refine this assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukemias are a heterogeneous group of neoplasms derived from hematopoietic cells. Disruption of the normal hierarchy of maturation results in hematological disorders characterized by either excesses or deficiencies of the mature effector cells [1]. Classification of leukemias is broadly related to the cell of origin (e.g., lymphoid or myeloid) as well as to the rapidity of the clinical course (e.g., acute or chronic) but modern categorizations have identified specific leukemias on the basis of biologic, antigenic, and molecular characteristics of these diseases [2, 3].
The causes of leukemia remain largely unknown although several factors have been found associated. They can be broadly grouped into (a) familial and genetic factors including inherited diseases (e.g., Down syndrome) or other chromosomal abnormalities (e.g., Philadelphia chromosome), (b) environmental factors resulting from occupational exposures, ionizing and non-ionizing radiation, chemicals, pesticides, smoking, diet, and other lifestyle factors as well as (c) medical- and therapy-related factors including radiotherapy, diagnostic irradiation, pharmaceuticals and chemotherapy, viruses [4]. Major identified risk factors that contribute to leukemogenesis are ionizing radiation in high dose, benzene exposure and at least one virus (HTL-1) [5].
There are several arguments in favor of a role of occupational exposures in the development of leukemia: the male excess of leukemia, the consistent increase among older males in many geographic areas, the higher incidence of acute myeloid leukemia in developed countries and industrial metropolitan areas in the US, and the presence of known and suspected hazardous agents in the workplaces [6].
Environmental and occupational exposures to pesticides as a risk factor for hematopoietic tumors have been widely studied mainly among farmers and agricultural workers, in rural communities and in the pesticide manufacturing industry but the results have not been consistent. Most of the earlier studies reported risk for leukemia as a single entity or by classification that did not allow to distinguish specific leukemia categories, partly because of the historical nomenclature but also due to the limited number of cases in individual studies. Failure to take into consideration the diversity of leukemias may account for some inconsistent findings from early epidemiological studies and, moreover, could hide type-specific risk factors. The diversity of leukemias deriving from a variety of stem cells at different hierarchical levels of hematopoietic and lymphoid cell development is indeed likely to be associated with a variety of etiological mechanisms [7]. In addition, as some exposures appear to be related to a specific histological type of leukemia (e.g., benzene most clearly associated with AML [4, 8]), it can be assumed that a family of pesticides could be related to a specific leukemia cell type. As the varying forms of leukemias probably have different etiologies, it is important to have a more precise breakdown of these data to properly assess the results [9].
As myeloid leukemias are the most frequent forms among adults [10], this paper focused on available epidemiologic data dealing these forms of the disease and occupational pesticide exposure to examine whether it is possible to obtain a more accurate estimate of their relationship.
Occupational exposure to pesticides includes a broad range of occupational categories such as end-users (e.g., farmers and applicators) and workers during the manufacturing process (manufacturing workers) both undergoing diverse qualitative and quantitative exposures.
In spite of these variations which make comparisons difficult, we followed in the present paper, a meta-analytical approach to examine subgroups of studies classified by occupational categories to possibly point to settings with the highest risk.
Materials and methods
Study identification and selection
Study identification
A search on MEDLINE (National Library of Medicine, Bethesda, MD) was conducted for the period 1966 to 31 May 2006. The search strategy combined medical subject headings and key words including myeloid, myelogenous, leukemia, chronic, acute, granulocytic, pesticides, occupation, farmers, agriculture, pesticide applicators, manufacturing workers, with no restriction of publication type or publication date. Recent articles in occupational medicine and epidemiology journals were also scanned for relevant publications. Finally, the reference lists of the relevant publications identified were checked for additional studies, limiting the search to studies published in English in the open literature, in peer-reviewed journals. Published studies were used as they are likely to be more reliable than unpublished reports.
Study selection
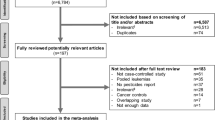
A study was considered eligible for further review if (1) it referred to an occupational group of interest exposed to pesticides (farmers, pesticide applicators, workers engaged in the manufacture of pesticides and others like horticulturists, greenhouse workers, gardeners, ...), (2) if the outcome included (subtypes of) myeloid leukemia in adults (International Classification of Diseases, 10th revision, code C92) [11] and (3) if the study used a cohort or case–control design. Excluded studies were those that did not report original results (reviews, comments, letters, editorials), experimental studies and proportional mortality ratios (PMR) studies (mainly because of ambiguities in interpreting results) as well as ecological studies (Fig. 1). Exceptions concerning occupational groups are studies on farmers’ wives or women living in a farm, also included in our analysis. Although not classified as occupationally exposed, they may be directly engaged in farm activities or be indirectly exposed to pesticides (e.g., household contamination).
Studies were also excluded if they concerned certain forms of pre-leukemia, known as myelodysplastic syndromes (International Classification of Diseases, 10th revision, code D46) [11] in which about 5–40% develop AML [12], if they included subjects already included in another more complete or more recent study examining a greater number of subjects or with longer follow-up duration and if they provided insufficient data to determine an estimator of relative risk (RR) for myeloid leukemia and its confidence interval.
The systematic review and identification of eligible studies was performed by 1 reviewer (GVMF).
Data extraction
A structured abstract was derived for each study identified. Two authors read the reports and independently extracted and tabulated the most relevant RR estimators, with their 95% CIs. The results of this exercise were compared between the authors and consensus was obtained before the meta-analysis. Mortality and incidence outcomes were combined as both are likely to be equally affected by the potential causal factor.
Overall meta-analysis
Cohort studies: if more than one follow-up analysis had been published for the same population, we used the most recently published report. Generally, when multiple estimates of RR were given, we retained (or calculated, see below) the overall data for the total cohort, for any exposure, for the total follow-up period and for all types of myeloid leukemia. Exceptions were papers reporting data justifying their inclusion in other meta-analyses as detailed below (stratifying studies in the meta-analyses). We did not include data resulting from further stratification, e.g., by latency period, by gender, by job title/employee group, by exposure groups, by factory. In some cases, the RR and/or 95% CI was not reported in the publication but we could derive it from the raw data presented [13].
Case–control studies: no overall meta-analysis was performed for case–control studies because when studies reported multiple OR, the available data did not allow to calculate a combined OR e.g., for all types of ML or for both genders. These data were included in stratified meta-analyses.
Stratifying studies in the meta-analyses
Cohort studies: separate meta-analyses were conducted by stratifying data (a) for different occupational groups (manufacturing workers, pesticide applicators, and farmers/agricultural workers), (b) for different types of ML (AML and CML), (c) by gender (men and women) and by gender and ML subtype.
Case–control studies: stratifications were performed for the different types of ML, for occupation (farmers/agricultural workers or occupationally exposed to pesticides when further specification was lacking) and for gender and ML subtype.
Cohort and case–control studies: grouping of cohort and case–control estimates of relative risk was made for ML subtype, for occupation and ML subtype as well as for gender and ML subtypes.
Data analysis
A detailed account of the procedure for data analysis has been published before [14]. In brief, homogeneity among data was evaluated to test between-study comparability. The significance of the between-study variance was evaluated with the ln(RR) statistic test, which has a χ2 distribution with degrees of freedom equal to the number of studies pooled minus 1. The applied formula is: χ2 = Σ w i [ln(RR) i − ln(RR)p]2, for i = 1 to N, where N is the number of studies combined, RRp is the overall pooled RR estimate, RR i is the RR for the ith study and w i = 1/V i where V i is the variance of the ln(RR) i . A low p value for this statistic indicates the presence of heterogeneity, which questions the validity of the pooled estimates [15, 16].
In the absence of heterogeneity, we calculated RRs and CIs according to a fixed model [17], which assumes that results across studies differ only by sampling error. The study variance (V i ) was calculated, using the CI given, according to the equation V i = [(ln(CIupper) − ln(CIlower))/3.92]2. As detailed by Stewart et al. [18] and Dennis [19], the maximum likelihood estimate of the pooled RR in the fixed effect model is the exp(ln(RR)p). The pooled ln(RR)p equals Σ [ln(RR) i /V i ]/[Σ(1/V i )], where V i is the variance for an individual study as described above and ln(RR) i is the log RR estimate for study i. This is a variance-weighted least square mean. The variance of the pooled ln(RR)p, Var(ln(RR)p) or V p is given by: [SE(ln(RR)p)] = [Σ(1/V i )]−1 where SE is the standard error. The pooled variance is used to calculate a 95% CI around the pooled RR estimate.
When data are heterogeneous or if there is reason to believe that publication bias exists, the random effects model is more appropriate. Under this model, the point estimate of the pooled effect measure and its CI incorporate the additional variability due to between-study variance (τ2). Random effects models were applied, using the method described by Der Simonian and Laird [20]. These authors proposed a non-iterative estimator of τ2 defined as est(τ2) = max{0, [Q − (k − 1)]/[Σ w i − (Σ (w 2 i ))/Σ w i } where Q is the heterogeneity statistic, k is the total number of studies, and w i are the inverse variance weights for ln(RR). Potential sources of heterogeneity were evaluated by subset analysis.
The overall meta-analysis for cohort studies is represented by a forest plot where the confidence interval for each study is represented by a horizontal line and the point estimate by a square. The size of the square corresponds to the weight of the study in the meta-analysis. The confidence interval for the total is represented by a diamond.
The influence of study size was explored by plotting the natural logarithm of the estimate of RR (ln RR) versus the inverse of standard error (1/SE). Funnel plot asymmetry was tested by the linear regression method suggested by Egger et al. [21].
We conducted sensitivity analyses by omitting studies reporting imprecise values (weight < 1.5 and < 10%, respectively) to estimate the importance of individual studies in the combined summary statistic and to determine whether any of these had a disproportionate influence [22]. We also conducted influence analyses to reestimate the pooled relative risk while dropping 1 study at a time and examine whether any studies disproportionately influenced the results.
Results
Literature selection and study characteristics
A large number of articles were retrieved from MEDLINE and hand searching in the reference lists of the relevant publications. We reduced these to a list of 251 studies selected for further evaluation (Fig. 1). Among these studies, 186 were excluded for the following reasons: no myeloid cell type (n = 110), no pesticide exposure and/or occupational group of interest (n = 15), other than cohort or case–control design (n = 61): reviews or meta-analyses (n = 24), case-reports or letters (n = 13), experimental studies and/or cytogenetic abnormalities (n = 16), PMR or ecologic studies (n = 8).
Among the 65 remaining eligible studies, 32 were excluded because they explored other disease conditions (e.g., myelodysplastic syndrome, thrombocytopenia, pre-leukemic condition, second primary cancer) but not ML (n = 9), the data were redundant/updated/overlapping (n = 9), data were insufficient to determine a risk estimate and its confidence interval (n = 14). Seventeen cohort studies [23–39] and 16 case–control studies [40–55] were identified as fulfilling the inclusion criteria.
Tables 1 and 2 provide selected characteristics of the cohort and case–control studies used in the analysis, respectively. The studies were published between 1979 and 2005. Studies differed according to the definition of cases, either restricted to AML (n = 17) and/or to CML (n = 12) or without subtype specification of ML (n = 12). Nine cohorts and 7 case–control studies were from Europe, 6 cohorts and 7 case–control studies from USA/Canada, 2 cohorts and 1 case–control studies from Australia/New Zealand and 1 case–control study was from China. Data were presented separately for women (n = 7) and for men (n = 19) and/or combined for both genders (n = 15). Pesticide exposed workers included manufacturing workers (2 cohort studies), pesticide applicators (5 cohort studies), farmers or agricultural workers (9 cohort and 12 case–control studies), horticulturists (1 cohort study) and occupational exposure to pesticides without other precisions (5 case–control studies). Reference populations in the cohort studies represented predominantly national, provincial or regional large populations. Eleven cohort and 3 case–control studies were mortality studies, 6 cohort and 12 case–control studies were morbidity studies and 2 case–control studies reported OR for mortality and incidence.
The estimates of the RR for the pesticide exposed groups of workers to develop or die from myeloid leukemia varied between 0.25 and 9.55 and included from 1 up to 458 cases. Thirteen studies (5 cohort and 8 case–control) reported increased RR estimators for AML with 2 cohort studies presenting a confidence interval that did not contain 1. Four case–control studies had relative risk estimates lower than 1. For CML, increased RR estimators were reported in 9 studies (3 cohort and 6 case–control) with 1 cohort reporting a 95% CI that did not include 1. Decreased CML relative risk estimators were reported in three case–control studies. Among the 10 studies (7 cohort and 3 case–control) reporting increased RR estimators for ML, 1 cohort and 2 case–control studies had a confidence interval that did not contain 1. Reduced risk was reported in 3 cohort and 1 case–control studies, 2 out of the 3 cohort studies presenting a 95% CI that did not include 1. One study reported no association between ML and occupational exposure of agricultural workers with a RR estimator of 1.
Data synthesis
Table 3 summarizes the results of the different meta-analyses of cohort and case–control studies performed.
Cohort meta-analyses
The meta-RR for all cohort studies was 1.21 (95% CI: 0.99–1.48). A forest plot of the 17 cohort studies is reported in Fig. 2. The study of Ji and Hemminki [31] contributed 54% of the total weight. Removal of this study from the overall meta-analysis resulted in a meta-rate ratio of 1.32 (95% CI: 1.01–1.72). No other studies contributed more than 30% of the total weight. The strong heterogeneity existing among the 17 RR estimates (p value of 1.06 × 10−5) argues against an overall meta-analysis of the data. Further analyses were therefore carried out to identify sources of heterogeneity, pooling studies according to different stratification variables.
Forest plot of cohort studies on myeloid leukemia among workers occupationally exposed to pesticides. Note. Estimators of RR and 95% confidence intervals (CIs) of cohort studies included in the overall meta-analysis are presented. Each estimator was assigned a weight (w i ) equal to the inverse square of its standard error (SE): w i = 1/(SE)2
Stratification of the studies by occupation indicated consistency among studies including manufacturing workers and pesticide applicators as evinced by p values for heterogeneity of 0.5 and 0.6, respectively, but not for farmers and/or agricultural workers (p value of 0.0008). For manufacturing workers (mRR: 6.32; 95% CI: 1.90–21.01) and pesticide applicators (mRR: 2.14; 95% CI: 1.39–3.31) relative risk estimators were statistically significantly increased.
Stratification of the studies by myeloid leukemia subtypes (chronic or acute) reduced heterogeneity but some variation across studies persisted as indicated by p values of 0.03 and 0.008, respectively. A significantly increased risk was observed for AML (mRR: 1.55; 95% CI: 1.06–2.26).
Stratification by gender (men or women) yielded no great difference between men (mRR: 1.08; 95% CI: 0.88–1.32) and women (mRR: 1.20; 95% CI: 0.84–1.74) and heterogeneity remained although reduced when compared with all cohort studies combined.
Crossing stratification by gender and myeloid leukemia subtypes strongly reduced heterogeneity for AML and CML in men (p values of 0.05 and 0.46, respectively). Meta-rate ratios higher than 1 were obtained but the confidence intervals included 1.
Case–control meta-analyses
Meta-RR were calculated after grouping studies by ML subtype (AML, CML, ML), by occupation and ML subtype (farmers/agricultural workers and AML or CML; occupational exposure to pesticides and AML or CML) as well as by gender and ML subtype, combined (men and CML; men and AML). Women data were to scarce to allow stratification of the case–control studies. No evidence of heterogeneity existed among the different RR estimators combined. The highest meta-RR estimators with confidence intervals not including one were observed for CML among farmers/agricultural workers and for CML in men. A borderline statistically significant increased meta-RR was observed for CML.
Cohort and case–control studies
Stratification of the studies by ML subtype, by ML subtype and occupation as well as by ML subtype and gender could be performed, resulting in consistency among studies for all groupings except for AML in women. All calculated meta-RR were higher than 1 but none reached statistical significance.
Six studies (3 cohort studies and 3 case-control studies) reported 7 RR estimators of ML according to high exposure to pesticides. These results are summarized in Table 4. In the three cohort studies, higher estimators of relative risk were observed for the high exposure groups, all of them showing confidence intervals not including 1. Among the case–control studies, 1 out of 4 estimators of relative risk increased, 2 were slightly decreased and 1 did not change.
Funnel plot and assymetry
The visual inspection of Fig. 3 suggests some asymmetry arising from a lack of small studies with low RR estimators. The linear regression method of Egger et al. [21] which confirmed the suggested asymmetry (intercept 2.489; 95% CI: 0.4769–4.501; p = 0.02).
Discussion
Despite the large number of publications dealing with pesticide exposure and leukemias in general, only a few epidemiological studies focused on specific subtypes of the disease. In most cases, the findings were based on a small number of events in each nosological category leading to non-significant and inconsistent results.
The present study is, to our knowledge, the first meta-analytical approach to assess pesticide exposure as a potential occupational risk factor for adult myeloid leukemia.
The results did not clearly indicate evidence of an overall increased risk. Although almost all calculated meta-RRs were higher than 1, only a few showed a clear excess risk. After stratifying the studies by occupational group, evidence of an increased risk emerged from the cohort studies for manufacturing workers and pesticide applicators. From the case–control studies an increased risk of CML emerged for the farmers/agricultural workers. After stratification of the studies by ML subtype, an increased risk emerged for AML in cohort studies as well as a borderline increase for CML in case–control studies. The apparent discrepancy between some of these results does not allow to conclude firmly with regard to the existence of a relationship between myeloid leukemia and overall occupational pesticide exposure. A causal interpretation of the increased risk of ML has generally not been suggested by the authors of the individual studies.
A salient argument in favor of a causal relationship between pesticide exposure and occurrence of ML was that the strongest meta-RRs were observed for both occupational groups (manufacturing workers and pesticide applicators) likely to have been more frequently and intensely exposed to pesticides. Although no heterogeneity was found in the effects measured among cohort studies specifically dealing with these occupational groups, it should, however, be kept in mind that qualitative diversity among studies existed with regard to the likely chemical exposures, the definition of the cohort, the exposure contrast, the comparison population, and the leukemia subtype. This did not allow to isolate a specific pesticide or a particular occupational group within pesticide applicators or manufacturing workers more likely to be related to a ML subtype. The results were obtained by pooling a small number of cohort studies and could not be confirmed by case–control studies but most of the latter concerned farmers/agricultural workers and not manufacturing workers or pesticide applicators.
An increased risk of ML could not be linked to a specific chemical class of pesticide. Manufacturing workers’ exposures were specific to alachlor [23] and phenoxy herbicides and chlorophenols [28]. Pesticide applicators handled a wider variety of pesticides (including chlorinated hydrocarbons, carbamates, organophosphates, phenoxyacetic acids, phthalimids, and coumarins) and pesticide use has also changed over the past several decades.
For causal inference it is also important to examine the risk according to a gradient of exposure. In the studies included in our analyses, data were available only for rough exposure-response analyses. Four studies presented exposure-response data using duration of exposure (years of employment, duration of occupational exposure) [25, 40, 44, 55], one study using exposure quartile [35] and one study using qualitative exposure ranking [23]. Duration of employment is often used as a surrogate of an increasing cumulative exposure potential. This assumes that exposure is uniform and continuous over the employment period, especially for pesticide exposure, but many circumstances challenge this assumption. The three cohort studies reported increased estimators of relative risk for high exposure as compared to corresponding results for any exposure.
There are also arguments against a causal interpretation of our results. Control of confounding by other occupational exposures and/or by non-occupational factors remains a concern as it is for virtually all studies in occupational settings. There are, however, few other known risk factors for ML (familial and genetic factors, environmental factors and medical and therapy related factors) and therefore few potential confounders that could have been controlled. Information on these risk factors was generally lacking in studies included in this meta-analysis.
Bias is also a serious concern in meta-analysis. We found evidence of funnel plot asymmetry among cohort studies (Fig. 3) which may lead to overestimate the real effect. The cohort studies that were included in the present meta-analysis did, however, not focus specifically on myeloid leukemia. Myeloid leukemia was reported among other cancer types as part of the results of specific population surveillance. As a consequence, the criticism of the meta-analysis method concerning the limited use of negative findings (less likely to be reported in peer reviewed sources) as well as the opposite criticism that positive studies of occupational hazards could be suppressed by economic forces are probably less relevant in the present meta-analysis. Furthermore, funnel plot asymmetry may have causes other than bias. A funnel plot should be seen as a mean of examining the tendency for the smallest studies of low quality in a MA to show larger effects [56]. As a consequence, a sensitivity analysis was conducted by removing the smallest study results with imprecise values (weight < 1.5% and weight < 10%, respectively) [data not shown]. The summary RR based on the remaining 7 and 3 large studies was 1.14 (95% CI: 0.86–1.27) and 0.87 (95% CI: 0.73–1.05), respectively, as compared with the RR of 1.21 (95% CI: 0.99–1.48) based on all the 17 cohort studies. The decreased summary RRs observed after removing studies with imprecise values suggests that “small study effects” can partially explain the borderline positive result of the MA of all cohort studies.
The influence of excluding each individual study on the pooled estimator of ML was examined. Pooled RRs obtained after removing each study in turn varied between 1.13 and 1.32. The magnitude of the effect observed varied, with the Sperati study appearing to be the most influential in terms of reduction in the point estimate for the pooled RR (1.13). The 95% CI became narrower when this study was removed because the between-study heterogeneity was reduced. Inference changed when other studies were removed, which was expected given that the initial result was already of borderline significance. A similar situation has been observed for leukemia among workers in the synthetic rubber-producing industry [57].
Exposure assessment is a critical component of epidemiologic research in occupational settings. It varied considerably among the studies included in the present MA. In an attempt to partially reduce this variability, studies were stratified by occupational categories although exposures within a same occupational group might also vary substantially.
The source to identify subjects as occupationally exposed to pesticides are reported in Tables 1 and 2 for cohort and case–control studies, respectively. The plant manufacturing alachlor [23] provided adequate job and department records to identify workers with specific pesticide exposure and the greatest confidence that workers were appropriately included. Manufacturing workers employed in plants producing or formulating a large number of chemicals [28] have a greater potential for misclassification of exposure. Pesticide applicators include various types of users (e.g., aerial pesticide applicators [29], licensed pesticide applicators [26, 37]), involved in different tasks (chemical brushwood control [24], structural pest control [26], agricultural chemicals spraying [29, 37, 39]). Workplace exposure was assessed by (job) records [24, 29] and by license or others registries [26, 37, 39]. Most cohorts of applicators used multiple classes of pesticides (e.g., chlorophenoxy herbicides, organophosphates, carbamates) and therefore contribute little information on the carcinogenic potential with respect to a specific pesticide or pesticide class but rather provide information regarding the health risk of the occupation [58]. Although studies of pesticide manufacturers or pesticide applicators tend to have more objective documentation of exposure (through exposure monitoring and job history), the diversity of exposure conditions cannot be taken into account because of the lack of detailed exposure data in the included studies. The problem is amplified in studies of occupational groups defined broadly as farmers or agricultural workers. Farm practices differ among regions, which leads to differences in type and degree of exposure. In the included studies, none of the farming variables directly measured exposure; all were surrogates. Exposure assessment methods were based on mortality or incidence registries [25, 27, 33, 34], censuses [30, 31], location of residence [36], individual reports [35] and farm membership [38] lists. These methods often suffer from a crude classification of exposure to potentially hazardous agents and potential for misclassification is a major limitation. Exposure overestimation is frequent and this misclassification may distort or dampen disease associations when there is a real underlying relationship between exposure and outcome. This may partially explain the lower risk observed for these occupational groups (Table 3).
All studies included workers ever employed in an occupation related to pesticide exposure but with different exposure assessments. An assumption that underlies our analyses is that all individuals included in these cohorts were exposed in their workplace.
The lack of adequate exposure definition and/or the variations in definitions of exposure across studies is a problem encountered in the vast majority of epidemiologic studies in occupational settings. Influential factors (like those related to pesticide application and behavior) should be incorporated into exposure estimates to reduce measurements error and to allow better resolution of the exposure distribution. Future work to develop exposure metrics that incorporate both chemical and exposure characteristics will contribute to improve these assessments [59].
Disease misclassification has also to be taken into consideration as the reporting of ML data in epidemiological studies has been variable. Most mortality studies refer to codes of the International Classification of Diseases (ICD). However, revisions of the ICD, periodically implemented according to advances in medical science, resulted in the introduction of more sophisticated coding systems. Thus, when ML data are reported, they may refer to some broader or narrower category. Specification of the ML subtypes (e.g., AML or CML) allows to partially reduce disease misclassification but this was made only by few authors. These difficulties with disease nomenclature contribute to obscure the relationship between leukemia subtype and occupational exposure to pesticides.
Conclusion
Our results from cohort studies indicate some evidence that exposure encountered by manufacturing workers and pesticide applicators increases the risk of ML among these occupational groups. Further studies that would correlate reliable exposure data for these groups with well-defined subtypes of leukemia are needed to confirm this finding. The apparent discrepancy observed between cohort and case–control studies underscores again the need for further studies focusing on well-defined ML subtypes.
References
Lee GR, Foerster J, Lukens J, Paraskevas F, Greer JP, Rodgers GM (1999) Wintrobe’s clinical hematology (Hardcover), 10th edn. Lippincott Williams & Wilkins, 2764 pp
Beers MH, Berkow R (1999) Leukemias. The Merck manual of diagnosis and therapy, 17th edn. Merck & Co. Inc., Merck Research Laboratories, Whitehouse Station, NJ, USA
Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292–2302
Zeeb H, Blettner M (1998) Adult leukaemia: what is the role of currently known risk factors?. Radiat Environ Biophys 36:217–228
Greaves MF (1997) Aetiology of acute leukaemia. Lancet 349:344–349
Linet MS, Cartwright RA (1996) The leukemias. In: Schottenfeld D, Fraumeni JF Jr (eds) Cancer epidemiology and prevention, 2nd edn. Oxford University Press, NewYork, NY, pp 841–879
Greaves MF (2004) Biological models for leukaemia and lymphoma. IARC Sci Publ 157:351–372
Schnatter AR, Rosamilia K, Wojcik NC (2005) Review of the literature on benzene exposure and leukemia subtypes. Chem Biol Interact 153–154:9–21
Greenberg RS, Mandel J, Pastides H, Britton NL, Rudenko L, Starr TB (2001) A meta-analysis of cohort studies describing mortality and cancer incidence among chemical workers in the United States and Western Europe. Epidemiology 12:727–740
Smith MT, Zhang L (1998) Biomarkers of leukemia risk: benzene as a model. Environ Health Perspect 106:937–946
International Classification of Disease (1989). 10th revision. http://www.wolfbane.com/icd/index.html
Greenberg PL, Young NS, Gattermann N (2002) Myelodysplastic syndromes. Hematology (Am Soc Hematol Educ Program) 136–161
Breslow NE, Day NE (1987) Statistical methods in cancer research: the design and analysis of cohort studies. IARC scientific publications vol II, no 82. International Agency for Research on Cancer, Lyon
Van Maele-Fabry G, Willems JL (2003) Occupation related pesticide exposure and cancer of the prostate: a meta-analysis. Occup Environ Med 60:634–642
Clarke M, Oxman AD (eds) (2000) Cochrane reviewers’ handbook 4.1 (updated June 2000). In: Review Manager (RevMan) (computer program). Version 4.1. The Cochrane Collaboration 2000, Oxford, England
Lipsett M, Campleman S (1999) Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am J Public Health 89:1009–1017
Greenland S (1998) Meta-analysis. In: Rothman KJ, Greenland S (eds) Modern epidemiology, 2nd edn. Lippincott-Raven, Philadelphia, pp 643–673
Stewart RE, Dennis LK, Dawson DV, Resnick MI (1999) A meta-analysis of risk estimates for prostate cancer related to tire and rubber manufacturing operations. J Occup Environ Med 41:1079–1084
Dennis LK (2000) Meta-analysis for combining relative risks of alcohol consumption and prostate cancer. Prostate 42:56–66
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Olkin I (1994) Re: “A critical look at some popular meta-analytic methods”. Am J Epidemiol 140:297–299
Acquavella JF, Delzell E, Cheng H, Lynch CF, Johnson G (2004) Mortality and cancer incidence among alachlor manufacturing workers 1968–99. Occup Environ Med 61:680–685
Asp S, Riihimaki V, Hernberg S, Pukkala E (1994) Mortality and cancer morbidity of Finnish chlorophenoxy herbicide applicators: an 18-year prospective follow-up. Am J Ind Med 26:243–253
Beard J, Sladden T, Morgan G, Berry G, Brooks L, McMichael A (2003) Health impacts of pesticide exposure in a cohort of outdoor workers. Environ Health Perspect 111:724–730
Blair A, Grauman DJ, Lubin JH, Fraumeni JF Jr (1983) Lung cancer and other causes of death among licensed pesticide applicators. J Natl Cancer Inst 71:31–37
Bucchi L, Nanni O, Ravaioli A, Falcini F, Ricci R, Buiatti E, Amadori D (2004) Cancer mortality in a cohort of male agricultural workers from northern Italy. J Occup Environ Med 46:249–256
Bueno de Mesquita H, Doornbos G, Van der Kuip D, Kogevinas M, Winkelmann R (1993) Occupational exposure to phenoxy herbicides and chlorophenols and cancer mortality in The Netherlands. Am J Ind Med 23:289–300
Cantor KP, Silberman W (1999) Mortality among aerial pesticide applicators and flight instructors: follow-up from 1965–1988. Am J Ind Med 36:239–247
Dean G (1994) Deaths from primary brain cancers, lymphatic and hematopoietic cancers in Agricultural Workers in the Republic of Ireland. J Epidemiol Community Health 48:364–368
Ji JG, Hemminki K (2005) Occurrences of leukemia subtypes by socioeconomic and occupational groups in Sweden. J Occup Environ Med 47:1131–1140
Kelleher C, Newell J, MacDonagh-White C, MacHale E, Egan E, Connolly E, Gough H, Delaney B, Shryane E (1998) Incidence and occupational pattern of leukaemias, lymphomas, and testicular tumours in western Ireland over an 11 year period. J Epidemiol Community Health 52:651–656
Littorin M, Attewell R, Skerfving S, Horstmann V, Moller T (1993) Mortality and tumour morbidity among Swedish market gardeners and orchardists. Int Arch Occup Environ Health 65:163–169
Nanni O, Ravaioli A, Bucchi L, Falcini F, Ricci R, Buiatti E, Amadori D (2005) Relative and absolute cancer mortality of women in agriculture in northern Italy. Eur J Cancer Prev 14:337–344
Semenciw RM, Morrison HI, Morison D, Mao Y (1994) Leukemia mortality and farming in the Prairie Provinces of Canada. Can J Public Health 85:208–211
Sinner PJ, Cerhan JR, Folsom AR, Ross JA (2005) Positive association of farm or rural residence with acute myeloid leukemia incidence in a cohort of older women. Cancer Epidemiol Biomarkers Prev 14:2446–2448
Sperati A, Rapiti E, Settimi L, Quercia A, Terenzoni B, Forastiere F (1999) Mortality among male licensed pesticide users and their wives. Am J Ind Med 36:142–146
Stark AD, Chang HG, Fitzgerald EF, Riccardi K, Stone RR (1990) A retrospective cohort study of cancer incidence among New York State Farm Bureau members. Arch Environ Health 45:155–162
t' Mannetje A, McLean S, Cheng S, Boffetta P, Colin D, and Pearce N (2005) Mortality in New Zealand workers exposed to phenoxy herbicides and dioxins. Occup Environ Med 62:34–40
Adegoke OJ, Blair A, Shu XO, Sanderson M, Jin F, Dosemeci M, Addy CL, Zheng W (2003) Occupational history and exposure and the risk of adult leukemia in Shanghai. Ann Epidemiol 13:485–494
Bjork J, Albin M, Welinder H, Tinnerberg H, Mauritzson N, Kauppinen T, Stromberg U, Johansson B, Billstrom R, Mikoczy Z, Ahlgren T, Nilsson PG, Mitelman F, Hagmar L (2001) Are occupational, hobby, or lifestyle exposures associated with Philadelphia chromosome positive chronic myeloid leukaemia? Occup Environ Med 58:722–727
Blair A, Thomas TL (1979) Leukemia among Nebraska farmers: a death certificate study. Am J Epidemiol 110:264–273
Blair A, White DW (1981) Death certificate study of leukemia among farmers from Wisconsin. J Natl Cancer Inst 66:1027–1030
Brown LM, Blair A, Gibson R, Everett GD, Cantor KP, Schuman LM, Burmeister LF, Van Lier S, Dick F (1990) Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res 50:6585–6591
Brownson RC, Reif JS (1988) A cancer registry-based study of occupational risk for lymphoma, multiple myeloma and leukaemia. Int J Epidemiol 17:27–32
Burmeister L, van Lier SF, Isacson P (1982) Leukemia and farm practices in Iowa. Am J Epidemiol 115:720–728
Ciccone G, Mirabelli D, Levis A, Gavarotti P, Rege CG, Davico L, Vineis P (1993) Myeloid leukemias and myelodysplastic syndromes: chemical exposure, histologic subtype and cytogenetics in a case–control study. Cancer Genet Cytogenet 68:135–139
Flodin U, Fredrikson M, Persson B, Hardell L, Axelson O (1986) Background radiation, electrical work, and some other exposures associated with acute myeloid leukemia in a case-referent study. Arch Environ Health 41:77–84
Gajewski AK, Krzyzanowski M, Majle T (1989) Occupational exposures and leukemia. Rocz Panstw Zakl Hig 40:1–5
Järvisalo J, Tola S, Korkala M-L, Järvinen E (1984) A cancer register-based case study of occupations of patients with acute myeloid leukemia. Cancer 54:785–790
Mele A, Szklo M, Visani G, Stazi MA, Castelli G, Pasquini P, Mandelli F (1994) Hair dye use and other risk-factors for leukemia and pre-leukemia: a case–control study. Am J Epidemiol 139:609–619
Reif J, Pearce N, Fraser J (1989) Cancer risks in New Zealand farmers. Int J Epidemiol 18:768–774
Richardson S, Zittoun R, Bastuji GS, Lasserre V, Guihenneuc C, Cadiou M, Viguie F, Laffont FI (1992) Occupational risk factors for acute leukaemia: a case–control study. Int J Epidemiol 21:1063–1073
Speer SA, Semenza JC, Kurosaki T, Anton-Culver H (2002) Risk factors for acute myeloid leukemia and multiple myeloma: a combination of GIS and case–control studies. J Environ Health 64:9–16
Terry PD, Shore DL, Rauscher GH, Sandler DP (2005) Occupation, hobbies, and acute leukemia in adults. Leuk Res 29:1117–1130
Sterne JA, Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 323:101–105
Alder N, Fenty J, Warren F, Sutton AJ, Rushton L, Jones DR, Abrams KR (2006) Meta-analysis of mortality and cancer incidence among workers in the synthetic rubber-producing industry. Am J Epidemiol 164:405–420
Burns C (2005) Cancer among pesticide manufacturers and applicators. Scand J Work Environ Health 31(suppl 1):9–17
Hoppin JA (2005) Integrating exposure measurements into epidemiologic studies in agriculture. Scand J Work Environ Health 31(suppl 1):110–114
Acknowledgments
V.M.F.G. was supported by a grant from the Belgian Ministry of Health (Grant Number 95/15 (164)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Maele-Fabry, G., Duhayon, S. & Lison, D. A systematic review of myeloid leukemias and occupational pesticide exposure. Cancer Causes Control 18, 457–478 (2007). https://doi.org/10.1007/s10552-007-0122-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-007-0122-2