Abstract
Purpose
We aimed to determine the prognosis and potential benefit of postoperative chemotherapy according to subtype of medullary breast carcinoma (MedBC), a very rare invasive breast cancer.
Methods
A cohort of 1518 female patients with unilateral MedBC and 284,544 invasive ductal carcinoma (IDC) cases were enrolled from the Japanese Breast Cancer Registry. Prognosis of MedBC was compared to IDC among patients with estrogen receptor (ER)-negative and HER2-negative subtype (553 exact-matched patients) and ER-positive and HER2-negative subtype (163 MedBC and 489 IDC patients via Cox regression). Disease free-survival (DFS) and overall survival (OS) were compared between propensity score-matched adjuvant chemotherapy users and non-users with ER-negative and HER2-negative MedBC.
Results
Among ER-negative and HER2-negative subtype patients, DFS (hazard ratio (HR) 0.45; 95% confidence interval (95% CI), 0.30–0.68; log-rank P < 0.001) and OS (HR 0.51; 95% CI 0.32–0.83; log-rank P = 0.004) were significantly better in MedBC than IDC. Patients treated with postoperative chemotherapy showed better DFS (HR 0.27; 95% CI 0.09–0.80; log-rank P = 0.02) and OS (HR 0.27; 95% CI 0.09–0.80; log-rank P = 0.02) compared to those without. For the ER-positive and HER2-negative subtype, the point estimate for HR for DFS was 0.60 (95% CI 0.24–1.22) while that for OS was 0.98 (95% CI 0.46–1.84) for MedBC.
Conclusion
In ER-negative and HER2-negative MedBC, the risk of recurrence and death was significantly lower than that of IDC, about half. Postoperative chemotherapy reduced recurrence and mortality. ER-positive and HER2-negative MedBC may have a lower risk of recurrence compared to IDC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary breast cancer (BC) comprises heterogenous pathological subtypes. Medullary breast carcinoma (MedBC) is a rare subtype of invasive BC, accounting for less than 1% of primary BC. The Ridolfi criteria are generally applied for histopathologic diagnosis [1], which include: a predominantly syncytial growth pattern; microscopically completely circumscribed; absence of intraductal component; moderate to marked diffuse mononuclear stromal infiltrate; nuclear pleomorphism; and absence of microglandular features.
Most MedBC exhibit the triple-negative subtype defined by negative expression of estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) [2], and prognosis is generally poor. Despite such pathological features of MedBC, some studies reported more favorable prognosis of MedBC than invasive ductal carcinoma (IDC) [3, 4], while others reported similar survival [5,6,7]. Probably because ER-positive and HER2-negative (ER+HER2−) MedBC is relatively rare, its prognosis has not yet been compared to that of IDC.
As a systemic therapy for triple-negative BC, only chemotherapy reduces recurrence and improves survival. Some previous studies reported that chemotherapy improved overall survival (OS) of MedBC by multivariate analysis [8, 9], while others did not [4, 10]. The reason for these discrepancies is unclear, but may be partly explained by the small number of patients and inadequate statistical methodologies used.
We aimed to clarify the difference in prognosis between MedBC and IDC according to subtype by adjustment of adequate covariates with sufficient patients in a large nationwide cohort named the Japanese Breast Cancer Registry (JBCR) database which includes patient characteristics, treatment and outcome information. Using this database we investigated recurrence in addition to OS as outcome. We also assessed whether chemotherapy was associated with improved outcomes among patients with ER-negative and HER2-negative (ER−HER2−) MedBC, which is another important clinical question, using propensity score (PS)-matched analysis.
Patients and methods
Data source
The JBCR database contains clinical records from more than 600,000 primary BC patients from more than 800 institutions in Japan as of 2016 [11]. The Registration Committee of the Japanese Breast Cancer Society (JBCS) managed the registry with support from the Public Health Research Foundation (Tokyo, Japan) up to year 2011. The registry is currently governed by the Registration Committee of the JBCS and managed by National Clinical Database, a platform for nationwide clinical registries with more than 15 specialty societies participating in its governance. Affiliated institutes provide data for newly diagnosed primary BC patients through a web-based system covering patient demographics, clinicopathological characteristics, survival data, including local recurrence, distant metastases, BC-specific survival, OS and therapies, such as types of surgery, radiotherapy, chemotherapy, hormone therapy and anti-HER2 therapy. Nuclear grade has been collected since 2013. Prognosis is collected at 5 and 10 years after surgery.
Study patients
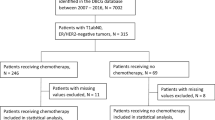
We utilized a cohort of patients with primary BC registered between 2004 and 2014 from the JBCR. We excluded males, patients with bilateral BC, and those without surgical treatment. The patients were then restricted to those with postoperative diagnosis of IDC or MedBC if they had no preoperative treatment, or those with preoperative diagnosis of IDC or MedBC if they had preoperative treatment (Fig. 1). To assess ER−HER2− patients and ER+HER2− patients, we further selected patients with these ER/HER2 statuses, and excluded those with preoperative systemic therapy as well as stage IV disease. The use of the data for retrospective observational studies was approved by the ethics committee of National Clinical Database, and the Ethics Review Committee at the JBCS approved the study [12].
Study outcomes
We assessed two survival endpoints: disease-free survival (DFS) and OS. DFS was defined as the time between surgery and local recurrence defined as either disease in the ipsilateral chest wall, skin or the ipsilateral axillary/supraclavicular/infraclavicular/internal mammary lymph nodes identified by biopsy and/or imaging, or distant metastases or death from BC. OS was defined as time from surgery until date of death from any cause.
Statistical analysis
We tabulated patients’ and tumor characteristics by pathological type, and also summarized perioperative treatments by type. We extracted the cohort of patients with ER−HER2− cancer and retabulated their background information by subtype. To compare the OS and DFS in MedBC against IDC ER−HER2− patients, we matched the MedBC patients to IDC patients at a 1:1 ratio according to their age, pT, pN, year of treatment and postoperative treatment including chemotherapy and radiotherapy, among those with prognosis information. We estimated the survival among the matched patients using the Kaplan–Meier (KM) method, and compared them using log-rank test. We also estimated hazard ratios (HR) for the two outcomes using Cox’s proportional hazards (PH) regression models.
To assess the association between chemotherapy and survival, we divided ER−HER2− MedBC patients into those treated with or without chemotherapy, and tabulated their main prognostic factors. We constructed a logistic regression model for predicting the receipt of chemotherapy in the cohort from these prognostic factors, and matched the chemotherapy patients to non-chemotherapy patients by nearest-neighbor matching without replacement with a caliper of 0.2 standard deviation of logit PS. Survival between the use and non-use groups were compared using KM curves as well as Cox models. We have also conducted a post hoc analysis to estimate the relative hazard of those undergoing chemotherapy compared with those not among pT1a-b patients.
Lastly, we extracted ER+HER2− patients from the overall (IDC and MedBC) cohort and tabulated the background factors between IDC vs. MedBC. Because the level of nuclear grading, an important outcome determinant, was greatly unbalanced between the IDC and MedBC, we imputed the value of the level of nuclear grading for subjects whose data was missing by multiple imputation method [13] to derive the HR from multivariable Cox models for the two outcomes, adjusting for the factors tabulated above. We applied fully conditional specification multiple imputation method via random forest using mice R package [14]. We set 50 as number of imputation dataset, and calculated the bootstrapped 95% confidence intervals for the estimated HRs (number of bootstrapping = 500).
Results
Patients comprised 1518 MedBC patients and 284,544 IDC patients (demographics shown in Table 1). Age, menopausal status, and body mass index distribution were very similar in the two groups, while clinical and pathological T or N stage were also comparable. The proportion of ER-negative tumors was greater in the MedBC group (72.8% vs 19.0%), while the proportion of HER2+ patients was similar in both groups. Tumors with nuclear grade 3 were also more frequent in the MedBC group.
Among the ER−HER2− patients, there were 785 patients with MedBC and 28,222 patients with IDC. Patient backgrounds were generally similar between the two groups, but the proportion of patients with nuclear grade 3 was higher in the MedBC group (85.6% versus 60.5%). The proportion of patients who received postoperative chemotherapy (66.6% versus 63.8%) was almost the same. We identified 17,652 IDC patients and 573 MedBC patients with follow-up information. After exact matching, we identified 553 patients with the same background regarding age, pathological T, pathological N, year of treatment and postoperative treatment including chemotherapy and radiotherapy. Background factors of the matched cohort were similar to the MedBC group in Table 1, including pathological factors and postoperative chemotherapy or radiation (Supplemental Table 1). KM curves for DFS and OS are shown in Fig. 2a and b, respectively, on MedBC and IDC patients with ER−HER2− subtype. The estimated 5-year DFS of ER−HER2− patients was 91.2% in MedBC and 82.1% in IDC (HR 0.45; 95% confidence interval (95% CI), 0.30 to 0.68; log-rank P < 0.001). The estimated 5-year OS was 91.3% in MedBC and 83.8% in IDC (HR 0.51; 95% CI, 0.32 to 0.83; log-rank P = 0.004).
Among the ER−HER2− patients with MedBC, those receiving post-operative chemotherapy were younger, included a greater proportion of pT2, and were more likely to be rated N1–2 (Table 2). The clinicopathologic characteristics of ER−HER2− patients with MedBC without post-operative chemotherapy before matching are shown in Supplemental Table 2 by pathologic tumor size. After PS matching, 153 patients remained in each group. This matched cohort included a higher proportion of patients over 65 years of age (44.1% vs. 31.1%) and pN0 (89.9% vs. 77.4%) compared to the original ER−HER2− MedBC cohort. KM curves for DFS and OS are shown in Fig. 3a and b, respectively. The estimated 5-year DFS of patients with and without postoperative chemotherapy was 95.7% and 89.0%, respectively (HR 0.27; 95% CI 0.09 to 0.80; log-rank P = 0.014). The estimated 5-year OS of patients with and without postoperative chemotherapy was 95.5% and 86.2%, respectively (HR 0.27; 95% CI 0.09–0.80; log-rank P = 0.003). A post hoc analysis among the subset of pT1a and pT1b MedBC patients showed that the hazard ratios in this subgroup is similar to those in the overall population (HR for DFS 0.22; 95% CI 0.07–0.67, HR for OS 0.30; 95% CI 0.11–0.83).
In the cohort of ER+HER2− patients there were 239 patients with MedBC and 168,844 patients with IDC (Table 3). Median age was the same in the two groups. Although the proportion of pathologically node-negative patients was higher, pathological tumor size was larger in MedBC. Among those with nuclear grade information, there was a marked imbalance, with 14.2% of IDC and 75.8% of MedBC being nuclear grade 3, similar to the ER−HER2− population. The proportion with negative progesterone receptor status was higher in MedBC. As described above, the pathological findings of MedBC have poorer prognostic features than those of IDC, which may have resulted in a higher proportion of MedBC patients being given post-operative chemotherapy (35.6% vs 24.1%). Among them, we identified 107,190 IDC patients and 167 MedBC patients with follow-up information. The HR from the Cox multivariate regression analysis in the multiple imputed dataset was 0.60 (95% CI 0.24–1.22) for DFS, and 0.98 (95% CI 0.46–1.84) for OS.
Discussion
Comparing patients with exactly matched background factors showed that the prognosis of ER−HER2− MedBC is better than IDC. We also investigated the ER+HER2− subtype, which constitutes a minority of MedBC, suggesting that there was a trend toward better DFS in MedBC at a similar extent to ER−HER2− cases, a finding not reported previously. Postoperative chemotherapy was shown to reduce death as well as recurrence by 73% each in ER−HER2− MedBC patients using PS-matched patients.
Several studies have compared prognosis between MedBC and IDC and reached different conclusions. Huober et al. used data from 13 clinical trials including 127 patients with MedBC and 8,096 patients with IDC and concluded that DFRI of MedBC was better than IDC in the full cohort (HR 0.52 95% CI; 0.36–0.75, P = 0.0005) as well as in the cohort with ER-negative and nuclear grade 3 (HR 0.24; 95% CI, 0.10–0.58, P = 0.002) [3]. Unfortunately, background factors were not adjusted, and multivariate analysis was not conducted, thus confounding factors could have affected the results. Another weakness was the lack of information regarding HER2 status. Two other studies evaluated survival differences using the SEER database. Wang et al. reported similar prognosis between MedBC and IDC using 309 cases of MedBC and 84,455 of IDC from the SEER 18 database, but the number of events on BC-specific survival and OS was only two and three, respectively, indicating the lack of statistical power to detect any differences [7]. Dai et al. reported that the prognosis of MedBC was better than IDC using a patient cohort with coarsened exact matching between MedBC and IDC by matching all the included variables [4]. Five-year cumulative incidence of death from cancer for MedBC of 0.054 was significantly better than that of IDC of 0.07 (P < 0.001). However, the 5-year cumulative incidence of death from other causes for MedBC of 0.028 was also better than that of IDC of 0.035 (P = 0.061). Because the magnitude of the reduction in incidences of death from cancer and death from other causes was similar, the difference between MedBC and IDC observed in this study may only represent the bias, making the conclusion unconvincing. In the current study, we used exact matching to balance several background factors and minimize confounding. The estimated risk of recurrence or death of ER−HER2− MedBC can be reliably estimated to be about half that of IDC. Furthermore, we reported for the first time that MedBC may have a better prognosis than IDC in ER+HER2− subtype, to a similar extent to that in the ER−HER2− subtype. Although the distribution of nuclear grades differs markedly between MedBC and IDC in the ER+HER2− subtype, our database lacked data on nuclear grades. Therefore, we used the multiple imputation method to match the nuclear grades and were able to partially compensate for this weakness of our study.
The benefit of chemotherapy for MedBC has been examined by previous studies, with mixed results. Lim et al. carried out a multivariate analysis and reported that adjuvant chemotherapy significantly improved BCSS (P = 0.009) and OS (P = 0.007), but this benefit was limited for patients with larger tumors (> 2 cm) [9]. The major drawback of this study is that it included too few events, at only 14, to adjust 8 variables for multivariate analysis. The power to detect the benefit of chemotherapy on small tumors was also insufficient, and consequently their results suffered from overfitting and underpower. Mateo et al. also performed multivariate analysis and showed that patients with T1cN0M0 and T2N0M0 had improved OS if they received chemotherapy (HR for death, 0.40; 95% CI 0.26–0.62; P < 0.0005) [8]. Unfortunately, they employed the stepwise method which was unsuitable because it caused overfitting. Additionally, M1 patients who generally are not indicated for adjuvant chemotherapy were included in the analysis, HER2 status was missing in 71% of patients, and the data were not classified according to subtype. PS-matched analysis indicated that chemotherapy significantly reduced the risk of death (HR 0.50, 95% CI 0.32–0.77; P = 0.002), but again the data were not reported according to subtype. In contrast, Dai et al. reported by multivariate analysis that chemotherapy did not improve the prognosis of MedBC [4]. The caveat of this analysis is that chemotherapy did not improve the prognosis even in IDC, when it is known to improve prognosis. In addition, the data according to subtype was not available. Therefore, this result is also inconclusive.
The present study does not have such methodological difficulties. We therefore believe that our results are the most reliable at present. Postoperative chemotherapy for ER−HER2− MedBC can generally be recommended as it has been shown to more than halve the risk of recurrence or death. This, what stage patients with ER−HER2− MedBC are eligible for postoperative chemotherapy? Among triple-negative BC patients, adjuvant chemotherapy was found to be beneficial in node-negative pT1c but not in pT1a or pT1b patients [15]. The NCCN guidelines for BC recommended adjuvant chemotherapy for MedBC in the same way as IDC if the size is greater than 1 cm, i.e., T1c [16]. However, considering that the present study estimated the risk of recurrence and death for ER−HER2− MedBC to be about half that of IDC, the absolute benefit of postoperative chemotherapy for MedBC can be estimated to be about half that for IDC, and therefore the indication for chemotherapy should be considered very carefully. Node-negative pT1c patients with ER−HER2− MedBC might be considered candidates for no adjuvant chemotherapy in some cases.
The current study has limitations and strengths. First, data on histological grade before 2013, Ki-67 labeling index or comorbidities were not available, which may result in unmeasured confounding. Second, our data were not centrally reassessed for ER, PR, or HER2 status. On the other hand, the strength of our study is that it draws from more than 400,000 patients treated by qualified doctors and institutions in a ‘real-world’ setting and that it has internal and external validity. The JBCR covers more than 90% of BC patients currently diagnosed in Japan [17], thus we can study the data on recurrence in addition to survival, which the SEER or NCDB does not cover. Furthermore, we were able to obtain reliable data on ER−HER2− MedBC by applying adequate statistical methods to adjust available confounding factors in sufficient patients. We also were able to add new data to the literature on the prognosis of ER+HER2− MedBC which has not been reported.
In conclusion, in ER−HER2− MedBC, the risk of recurrence and death was significantly better than that of IDC, at about half. Postoperative chemotherapy reduces recurrence and mortality. ER+HER2−MedBC may have lower risk of recurrence compared to IDC.
References
Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V (1977) Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer 40:1365–1385. https://doi.org/10.1002/1097-0142(197710)40:4%3c1365::aid-cncr2820400402%3e3.0.co;2-n
Park I, Kim J, Kim M, Bae SY, Lee SK, Kil WH, Lee JE, Nam SJ (2013) Comparison of the characteristics of medullary breast carcinoma and invasive ductal carcinoma. J Breast Cancer 16:417–425. https://doi.org/10.4048/jbc.2013.16.4.417
Huober J, Gelber S, Goldhirsch A, Coates AS, Viale G, Ohlschlegel C, Price KN, Gelber RD, Regan MM, Thurlimann B (2012) Prognosis of medullary breast cancer: analysis of 13 international breast cancer study group (IBCSG) trials. Ann Oncol 23:2843–2851. https://doi.org/10.1093/annonc/mds105
Dai D, Shi R, Wang Z, Zhong Y, Shin VY, Jin H, Wang X (2020) Competing risk analyses of medullary carcinoma of breast in comparison to infiltrating ductal carcinoma. Sci Rep 10:560. https://doi.org/10.1038/s41598-019-57168-2
Vo T, Xing Y, Meric-Bernstam F, Mirza N, Vlastos G, Symmans WF, Perkins GH, Buchholz TA, Babiera GV, Kuerer HM, Bedrosian I, Akins JS, Hunt KK (2007) Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg 194:527–531. https://doi.org/10.1016/j.amjsurg.2007.06.012
Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW (1992) Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 20:479–489. https://doi.org/10.1111/j.1365-2559.1992.tb01032.x
Wang XX, Jiang YZ, Liu XY, Li JJ, Song CG, Shao ZM (2016) Difference in characteristics and outcomes between medullary breast carcinoma and invasive ductal carcinoma: a population based study from SEER 18 database. Oncotarget 7:22665–22673. https://doi.org/10.18632/oncotarget.8142
Mateo AM, Pezzi TA, Sundermeyer M, Kelley CA, Klimberg VS, Pezzi CM (2017) Chemotherapy significantly improves survival for patients with T1c–T2N0M0 medullary breast cancer: 3739 cases from the national cancer data base. Ann Surg Oncol 24:1050–1056. https://doi.org/10.1245/s10434-016-5649-6
Lim S, Park SH, Park HK, Hur MH, Oh SJ, Suh YJ (2015) Prognostic role of adjuvant chemotherapy in node-negative (N0), triple-negative (TN), medullary breast cancer (MBC) in the Korean population. PLoS ONE 10:e0140208. https://doi.org/10.1371/journal.pone.0140208
Trapani D, Giugliano F, Uliano J, Zia VAA, Marra A, Viale G, Ferraro E, Esposito A, Criscitiello C, D’amico P, Curigliano G (2021) Benefit of adjuvant chemotherapy in patients with special histology subtypes of triple-negative breast cancer: a systematic review. Breast Cancer Res Treat 187:323–337. https://doi.org/10.1007/s10549-021-06259-8
Kubo M, Kumamaru H, Isozumi U, Miyashita M, Nagahashi M, Kadoya T, Kojima Y, Aogi K, Hayashi N, Tamura K, Asaga S, Niikura N, Ogo E, Iijima K, Tanakura K, Yoshida M, Miyata H, Yamamoto Y, Imoto S, Jinno H (2020) Annual report of the Japanese breast cancer society registry for 2016. Breast Cancer 27:511–518. https://doi.org/10.1007/s12282-020-01081-4
Miyata H, Gotoh M, Hashimoto H, Motomura N, Murakami A, Tomotaki A, Hirahara N, Ono M, Ko C, Iwanaka T (2014) Challenges and prospects of a clinical database linked to the board certification system. Surg Today 44:1991–1999. https://doi.org/10.1007/s00595-013-0802-3
Tsiatis AA (2006) Semiparametric theory and missing data. Springer, New York
Doove LL, Van Buuren S, Dusseldorp E (2014) Recursive partitioning for missing data imputation in the presence of interaction effects. Comput Stat Data Anal 72:92–104. https://doi.org/10.1016/j.csda.2013.10.025
Steenbruggen TG, van Werkhoven E, van Ramshorst MS, Dezentjé VO, Kok M, Linn SC, Siesling S, Sonke GS (2020) Adjuvant chemotherapy in small node-negative triple-negative breast cancer. Eur J Cancer 135:66–74
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Matro J, Mayer IA, Mortimer J, O'Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns JL, Kumar R (2021) NCCN guidelines® insights: breast cancer, version 4.2021
Tokuda Y, Kumamaru H, Jinno H (2019) The Japanese breast cancer society breast cancer registry in the national clinical database: current status and future perspectives to improve outcomes for breast cancer patients. J Jpn Surg Soc 120:639–645 (in Japanese)
Acknowledgements
We thank the affiliated institutes participating in the Japanese Breast Cancer Registry of the JBCS for their efforts to register patients’ data. We thank the Japanese Breast Cancer Society for their grant support to perform the present study.
Funding
This work was supported by the Japanese Breast Cancer Society.
Author information
Authors and Affiliations
Contributions
The study was designed by TA, HK, MI, and MM. All data were analyzed by HK, HM, YT and interpreted by all authors. SI and HJ contributed to funding acquisition and supervision. TA, MI, and MM drafted the manuscript. All authors listed, critically reviewed, and approved the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
HK reports receiving speaker fees from Chugai Pharmaceutical Co., Ltd and Johnson and Johnson KK, and consultation fees from Mitsubishi Tanabe Pharma and EP Croit Co., Ltd. HK is affiliated with the department of Healthcare Quality Assessment at the University of Tokyo. The department is a social collaboration department supported by National Clinical Database, Johnson and Johnson KK, Nipro corporation and Intuitive Sàrl. YT reports receiving consultant fees from Pharmaceuticals and Medical Devices Agency and EPARK, Inc., and lecture fees from SAS Institute Japan Ltd. YT and HK are conducting a collaborative study with Pfizer inc., which is not related to the topic of this study.
Ethical approval
This is a database study. The use of the data for retrospective observational studies was approved by the ethics committee of National Clinical Database, and the Ethics Review Committee at the JBCS approved the study.
Enquiries about data availability should be directed to the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aihara, T., Kumamaru, H., Ishitobi, M. et al. Prognosis and effectiveness of chemotherapy for medullary breast carcinoma. Breast Cancer Res Treat 196, 635–645 (2022). https://doi.org/10.1007/s10549-022-06749-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06749-3