Abstract
Purpose
Older cancer patients are underrepresented in clinical trials. We aimed to evaluate the enrollment of older women aged 70 years old (yo) or over with metastatic breast cancer (MBC) in clinical trials.
Methods
We used the national Epidemio-Strategy and Medical Economics MBC Data Platform, a French multi-center real-life database. We selected MBC women over 70yo, without central nervous system metastases, with at least one line of systemic treatment, between January 1st, 2008 and December 31st, 2016, and had no other cancer in the 5 years before MBC. The primary objective was to evaluate the proportion of patients enrolled in clinical trials according to their age. Secondary objective was to identify variables associated with enrollment in older ones.
Results
5552 women were aged ≥ 70 (median 74yo; IQR 72–77). 14,611 were less than 70. Of the older ones, 239 (4%) were enrolled in a clinical trial during first line of treatment, compared with 1529 (10.5%) for younger ones. Multivariable analysis of variables predicting for enrollment during first line of treatment in older patients were younger age (OR 0.50 [95%CI 0.33–0.76] for the 80–85yo class; OR 0.17 [95%CI 0.06–0.39] for the 85yo and more class), good ECOG Performance Status (PS 0–1) (OR 0.15 [95%CI 0.08–0.27] for the PS 2–4 class), HER2 + disease (OR 1.78 [95%CI 1.27–2.48]), type of treatment (chemotherapy/targeted therapy/immunotherapy OR 5.01 [95%CI 3.13–8.18]), and period (OR 1.65 [95%CI 1.22–2.26] for 2012–2016, compared to 2008–2011).
Conclusion
In this large database, few older MBC patients were enrolled in a trial compared with younger ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with an increased risk of cancer. In western countries, around 45% of breast cancer cases occur in women aged 65 years old or over and 20% in women over 75 years old [1]. Despite the recent advances in oncology treatments, older patients with metastatic breast cancer (MBC) still have poorer overall survival compared to their younger counterparts [2]. This might be due to later diagnosis, absence of screening, lack of knowledge of symptoms, neglect of clinical signs, physical and psychosocial barriers, or occult disease presentation. Older patients display particularities such as changes in organ function associated with increasing age that can alter absorption, distribution, metabolism, or excretion of drugs [3, 4]. They also present more acute organ dysfunctions, due to more comorbidities, illustrating the concept of frailty [5]. Moreover, older patients with metastatic disease are underrepresented in clinical trials [6,7,8,9,10,11] with no progress in the last years [12]. They may present different efficacy and/or toxicity profiles compared with younger adults that can affect outcomes [13, 14]. One recent study showed that the median age at inclusion in clinical trials of patients with breast cancer was 7.76 years [7.24, 8.28] younger than the median age at diagnosis [6]. This underrepresentation led to therapeutic guidelines that may be inappropriate, or at least not personalized enough, for older patients.
Successively, the FDA and the EMA issued guidance for including an adequate representation of older adults in cancer clinical trials to better define toxicity and efficacy in this population. They emphasize on the critical need to include adults over 75 years old especially in early phase trials to better extrapolate the results to the general population and have early pharmacokinetic and toxicity data [15, 16]. A recent position paper [17] of the International Society of Geriatric Oncology (SIOG) classified as one of their 12 priority axes, a large participation of older patients in clinical research programs, as well as the development of research protocols dedicated to the older population. In this context, real-life cohorts may bring valuable insight to identify variables associated with enrollment of older patients with MBC in clinical trials. In this study, we aim to evaluate the proportion of patients aged 70 years old or over with MBC enrolled in clinical trials in first-line clinical trials in France in the past decade and to identify variables associated with enrollment in a French multicenter real-life cohort.
Methods
We used the national Epidemio-Strategy and Medical Economics (ESME) Metastatic Breast Cancer (MBC) Data Platform [18], a multi-center real-life database using a retrospective data collection process in 18 French Cancer Centers. This database compiles data from Patient’s Electronic medical records. Cases selected were adult patients with MBC whose first metastasis was treated between January 1st, 2008 and December 31st, 2016. In compliance with the authorization delivered by the French Data Protection agency to R&D Unicancer (Registration ID 1,704,113 and authorization NODE-2013.-117, NCT03275311), only aggregated statistical reports were provided.
We selected women who were 70 years old or over at the time of MBC diagnosis, who received at least one line of systemic treatment, who had no other (non-breast) cancer in the 5 years before MBC, and lack of ‘Central Nervous System’ (CNS) metastases. The metastatic disease was defined as de novo when the metastases were diagnosed synchronously or ≤ 6 months from diagnosis of the primary tumor; and as recurrent when the metastases were diagnosed > 6 months from the diagnosis of the primary tumor. MBC treatment strategy could include chemotherapy (CT), targeted therapy (TT), and endocrine therapy (ET). Four subtypes were defined according to endocrine receptor (HR, estrogen and progesterone receptors) and HER2 status: HR+HER2−, HER2+ (regardless of HR status), HR− /HER2 − (triple-negative, TN), and undetermined.
For the ESME MBC cohort, HER2 and HR statuses were derived from existing results about metastatic tissue sampling where available, or, if not available, from last sampling on early disease. Tumors were defined as HR positive if estrogen receptor or progesterone receptor expression was ≥ 10% (immunohistochemistry). HER2 immunohistochemical (IHC) score 3 + or IHC score 2 + with a positive fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) classified the cancer as HER2 positive (HER2 +). On the other hand, all cancers with an IHC score 0, 1 +, or 2 + with a negative FISH/CISH test, as well as patients with a negative FISH/CISH test without IHC information, were considered as HER2 negative (HER2 −). Cancers with an IHC score 2 + without FISH/CISH test information were considered as HER2 indeterminate. Type of metastasis at MBC diagnosis was grouped as ‘Not visceral’ (including only bones and/or nodes metastases) and ‘Visceral’ (metastases other than bones and nodes). Number of metastatic sites at MBC diagnosis was described as < 3 versus ≥ 3. Year of MBC diagnosis was dichotomized according to the cut-off year 2012 (2008–2011 versus 2012–2016).
‘Enrollment in a clinical trial’ was registered in ESME MBC cohort, regardless of the type (systemic treatment, local treatment, supportive care for instance) and the phase of the trial (which consists of interventional studies, with authorization from health authorities and signing of informed consent, involving human volunteers, in intend to add to the medical knowledge). We focused on the enrollment in a clinical trial during the first line of treatment in metastatic setting.
The primary endpoint was the proportion of patients enrolled in clinical trials during the first line of treatment according to their age (threshold 70 years old). The secondary endpoints were variables associated with inclusion in a clinical trial in older patients (versus non included older patients), comparison of overall survival of older patients included in a trial versus non included, proportion of older patients enrolled between 2008–2011 and 2012–2016.
Statistical analysis
Descriptive statistics were used to summarize patients’ initial characteristics at diagnosis of metastatic disease. They were compared between groups using Chi-2’s or Fisher’s exact test for categorical data and Student T-test or non-parametric Wilcoxon’s test for continuous data; a p value < 0.05 was considered statistically significant. The reverse Kaplan–Meier method was used to estimate the median follow-up duration, beginning at the date of diagnosis of metastatic disease.
For the secondary endpoint (variables associated with inclusion in a clinical trial in older patients), in order to estimate the relative contribution of each variable, we used a multivariable logistic model. Variables included in the analysis were age at MBC diagnosis (continuous variable or in classes according to the age distribution in patients aged 70 and over), time from primary cancer to MBC diagnosis (de novo/recurrent), metastatic sites at diagnosis, phenotype (HER2+, HR+HER2−, or TN), ECOG Performance status (0–1/2–4/not available), type of treatment (CT/TT/immunotherapy alone or with ET versus ET alone), years of MBC diagnosis (2008–2011 versus 2012–2016), and number in ESME database per center per year, also called “Center Size” (< 500; 500–800; > 800 new patients per year).
Overall survival (OS) was defined as time (in months) between MBC diagnosis and date of death (any cause) or censored to date of latest news. Survival curves for OS with associated log-rank tests were generated using the Kaplan–Meier method.
A multivariable Cox model was performed to fit the model on the above-mentioned variables. All variables significant at a 15% level were included in the final multivariable model. Hazard Ratios (HR) are presented with 95% confidence interval (CI). The effect of inclusion or non-inclusion in a clinical trial on survival was estimated with and without adjustment in a Cox model.
A p value of less than 0.05 was considered statistically significant.
All analyses were performed using R software (R Core Team (2017)).
Results
Patients’ characteristics
On the 22 463 patients of the ESME data-base, we selected 5552 women aged ≥ 70 yo and 14,611 women aged < 70 yo (Fig. 1). The median age in the older group was 77 yo (Q1–Q3; 73–82). Time from primary breast cancer diagnosis to MBC was 52 months in the older population. Comparisons of characteristics of patients, MBC, and first-line treatments according to enrollment in a clinical trial are presented in Table 1. Most of the older patients (67%) had an HR+HER2– disease, 52% had visceral metastases, and 82% had less than 3 metastatic sites at MBC diagnosis.
Inclusion in clinical trials
Among the older population, 239 (4%) patients were enrolled in a clinical trial in the first-line setting compared to 1529 (10.5%) younger patients. When considering all lines of treatment, 7% of older women with MBC were enrolled in a clinical trial (Supplementary Table S1). Of note, the median follow-up was 46.7 (95%CI; 45.1–49.3) months in older patients.
Median age at enrollment in first line in older patients was 74 yo [Q1–Q3; 72–77]. In first line, 0.3% of older patients were enrolled in phase I, 1.1% in phase II, and 1.8% in phase III trials. The phase of the trial was unknown in up to 40%.
The proportion of older patients with MBC enrolled in clinical trials in the first-line setting increased over time, with 172 older patients in the 2012–2016 period (5.6%), compared to 67 older patients (2.7%) in the 2008–2011 period. If considering all lines of treatment for enrollment in a clinical trial, the proportion of older patients enrolled also increased, with 244 older patients (8.0%) in the 2012–2016 period compared to 151 (6.1%) in the 2008–2011 period. This is summarized in Fig. 2.
Variables associated with enrollment in clinical trials
Multivariable analysis of variables associated with being enrolled in a first-line clinical trial for the older patients aged 70yo and over is presented in Table 2. Bivariate analysis is presented in Supplementary Table S2. Among older women, variables associated with participation in a first-line clinical trial in multivariable analysis were younger age (OR 0.50 [95%CI 0.33–0.76] for the 80–85yo class; OR 0.17 [95%CI 0.06–0.39] for the 85yo and more class), good ECOG Performance Status (PS 0–1) (OR 0.15 [95%CI 0.08–0.27] for the PS 2–4 class; OR 0.19 [95%CI 0.13–0.28] for unknown PS class), HER2+ disease (OR 1.78 [95%CI 1.27–2.48]), and type of treatment (chemotherapy/targeted therapy/immunotherapy OR 5.01 [95%CI 3.13–8.18]; endocrine therapy + chemotherapy/targeted therapy/immunotherapy OR 5.44 [95%CI 3.59–8.50]). Being treated in the 2012–2016 period (compared to 2008–2011) was associated with an increased probability of enrollment in a first-line clinical trial (OR 1.65 [95%CI 1.22–2.26]. Variables associated with enrollment of older patients in first-line endocrine therapy trials were similar, with a 75 years old threshold (OR 0.61 [95%CI 0.41–0.90]), instead of 80 years old in the general population of older patients enrolled in a first-line trial (Supplementary Table S3). Variables associated with enrollment of older patients in first line for chemotherapy/targeted therapy/immunotherapy trials were younger age (< 80 yo) (OR 0.49 [95%CI 0.30–0.77] for the 80–85yo class; OR 0.17 [95%CI 0.05–0.43] for the 85yo and more class), ECOG PS0 (OR 0.69 [95%CI 0.48–0.99] for PS1, OR 0.18 [95%CI 0.09–0.32] for PS 2–4, OR 0.22 [95%CI 0.15–0.33] for unknown PS; compared with PS0), HER2+ disease (OR 1.80 [95%CI 1.30–2.49]) and the 2012–2016 period (OR 1.55 [95%CI 1.13–2.15]). Triple negative (OR 0.58 [95%CI 0.35–0.93]) and unknown subtypes (OR 0.35 [95%CI 0.11–0.86]) were associated with less enrollment in chemotherapy trials in older patients (Supplementary Table S4).
Overall survival
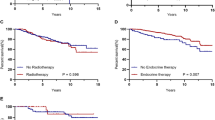
Median overall survival (OS) was 34.8 months in the older population (95%CI 33.6–36.0). Among older women, being enrolled in a trial in the first-line setting was associated with a better OS (HR = 0.78; 95%CI 0.63–0.95) in the multivariable analysis (Table 3 and Fig. 3).
Discussion
In this real-life French multicenter cohort, we found that only 4.3% of older women aged 70yo or more with MBC were included in a clinical trial in the first-line setting, compared to 10.5% for their younger counterparts. When considering all lines of treatment, 7.1% of older women with MBC were enrolled in a clinical trial.
Several studies [6,7,8,9,10,11,12] are consistent with our results showing that older patients were less frequently enrolled in clinical trials, but none of them was specifically dedicated to metastatic breast cancer patients. Moreover, some of these studies were focused on the age of the patients included in clinical trials compared to the median age of disease incidence [6,7,8, 12]. Other studies were centered on patients treated for gastro intestinal cancer [9], included a small number of patients [10], or used a threshold of 65 years old [11].
In a prospective multicentric French cohort study (Sujets AGés dans les Essais Cliniques—SAGE), evaluating enrollment of patients with colorectal cancer, the inclusion rate with a threshold of 70 yo or more was 9.5% [9]. In the SEER data-base including all tumor types [12], 20% of older patients aged 70 yo or more were enrolled in a clinical trial, far more than our population. As stated by the authors, this high level of enrollment was related to numerous adjuvant endocrine therapy programs, which can lead to more enrollment consistent with the better general condition of this population. The inclusion rate excluding those studies was 12%. Similarly, in Lackman’s study [11], enrollment rate was up to 14%, probably due to the high of proportion of non-metastatic patients (66%), and to a lower age threshold of 65 yo.
This low rate of enrollment in MBC was also observed in the OMEGA study, with several barriers to enrollment, such as patients’ refusal of chemotherapy or randomization, comorbidities, and oncologist’s preferences [19]. Other barriers to enrollment have also been described [11, 12, 20] such as availability of trials, older age, ECOG PS, but also the representations of patients and practitioners on the potential benefits and risks expected from the protocol on an individual basis.
The low inclusion rate in our cohort is possibly also related to the fact that the ESME database is a real-life cohort, with data from medical records. It is therefore quite possible that this inclusion rate is underestimated because of the retrospective nature of the data collection.
In our study, variables associated with participation in a clinical trial in the first-line setting in older patients were younger age (< 80 yo), good ECOG PS (0–1), HER2+ disease, and investigational treatment (chemotherapy/targeted therapy/immunotherapy trials). This is consistent with the SAGE and Lackman’s studies [9, 11]. In the SAGE study, older age (> 80 years old) was significantly associated with non-invitation to participate in a clinical trial (HR = 0.14; 95%CI 0.02–0.60).
The type of disease (HR+ etc.), and the trials available at that time, with their inclusion and exclusion criteria, are important confounding factors, which should lead to some caution in the analysis of these results. The fact that patients were less included in endocrine therapy alone trials is probably due to the few trials enrolling at that time. Conversely, patients harboring HER2+ disease were more often enrolled in clinical trials due to a larger range of clinical trials in this indication. Poor ECOG PS (> 1) is an usual exclusion criteria in trials, explaining our results with an OR of 0.15 in this patients’ population.
We also found a better overall survival in older MBC patients included during the first-line setting even after adjustments for confounding factors in a multivariable analysis. This can be interpreted in two ways: one is that patients included in clinical trials have a better natural prognosis and are selected for trials because of their better general condition. The other could be that having access to innovative treatments may increase life expectancy among these patients. Our data do not allow to identify the respective weight of these two potential complementary factors linked to a longer observed survival, because of the retrospective setting, and lack of certain data (unavailable ECOG PS values in almost 50% and unavailable comorbidities, geriatric assessments, and causes of death). Of note, a better overall survival when included in a clinical trial was also observed in the Korean MBC Database in a general MBC population [21].
Unfortunately, as mentioned, no geriatric data were available to better characterize older patients, such as cognitive status, depression, or functional autonomy, which could highly change the probability of enrollment in a clinical trial. The number of referrals, patient’s eligibility, and finally numbers of patients enrolled were not available in this database preventing from any conclusions regarding patient’s selection. Finally, due to its retrospective nature, data regarding study type (chemotherapy, supportive care, or radiotherapy for example), or study phase, was lacking in up to 40% in chemotherapy studies. The QUALISAGE study (NCT03230305), closed to enrollment, will probably help to answer these questions.
Finally, in our study, more patients were included during the 2012–2016 period compared to the 2007–2011 period. Efforts are still needed for a better representation and reliable guidelines [22, 23].
Conclusion
In this large real-life database, fewer older MBC patients were enrolled in a trial compared to younger ones. In older patients, variables associated with such participation to clinical research were younger age (< 80 yo), good ECOG Performance Status (0–1), HER2 + disease, and investigational treatment consisting of chemotherapy/targeted therapy/immunotherapy. Older patients were more enrolled in clinical trials between 2012 and 2016 compared to the 2008–2011 period (5.6% versus 2.7%). Most of these factors raise questions on drug availability and perceived potential benefits by investigators and medical teams. Accrual of older patients with cancer in MBC and other disease types should be more encouraged.
Data availability
The datasets analyzed during the current study are available in the Epidemio-Strategy and Medical Economics (ESME) Metastatic Breast Cancer (MBC) Data Platform. The database of the ESME program or the database of the MBC cohorts are currently not accessible. For any specific demand, please contact the corresponding author. Each demand will be examined on a case-by-case basis by the scientific committee.
Code availability
All analyses were performed using the software R. (R Core Team (2017).
References
National Cancer Institute. Surveillance, Epidemiology, and End result Program. https://seer.cancer.gov/statfacts/html/breast.html. Accessed 10 September 2020.
Deluche E, Antoine A (2020) Bachelot T Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicenter ESME cohort 2008–2016. Eur J Cancer 129:60–70. https://doi.org/10.1016/j.ejca.2020.01.016
Backer SD, Grochow LB (1997) Pharmacology of cancer chemotherapy in the older person. Clin Geriatr Med 13(1):169–183
Yuen GJ (1990) Altered pharmacokinetics in the elderly. Clin Geriatr Med 6(2):257–267
Clegg A, Young J,Iliffe, et al (2013) Frailty in elderly people. Lancet 381(9868):752–762. https://doi.org/10.1016/S0140-6736(12)62167-9
Ludmir EB, Mainwaring W, Lin TA et al (2019) Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2055
Trimble EL, Carter CL, Cain D et al (1994) Representation of older patients in cancer treatment trials. Cancer 74(7 Suppl):2208–2214
Le Saux O, Falandry C, Gan HK et al (2016) Inclusion of elderly patients in oncology clinical trials. Ann Oncol 27:1799–1804
Canouï-Poitrine F, Lièvre A, Dayde F et al (2019) Inclusion of older patients with cancer in clinical trials: the sage prospective multicenter cohort survey. Oncologist. https://doi.org/10.1634/theoncologist.2019-0166
Tack L, Lefebvre T, Lycke M et al (2020) Underrepresentation of vulnerable older patients with cancer in phase II and III oncology registration trials: A case-control study. J Geriatr Oncol 11:320–326
Lackman M, Vickers MM, Hsu T (2020) Physician-reported reasons for non-enrollment of older adults in cancer clinical trials. J Geriatr Oncol 11:31–36
Talarico L, Chen G, Pazdur R (2004) Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 22(22):4626–4631
Mariano C, Francl M, Pope J et al (2015) Comparison of toxicity experienced by older versus younger patients enrolled in breast cancer clinical trials. Clin Breast Cancer 15(1):73–79. https://doi.org/10.1016/j.clbc.2014.09.002
Wildiers H, Glas NA (2020) Anticancer drugs are not well tolerated in all older patients with cancer. Lancet Healthy Longev. 1(1):e43–e47
Inclusion of Older Patients in Cancer Clinical Trials. Guidance for Industry. FDA. March 2020. https://www.fda.gov/media/135804/download. Accessed 09 February 2021.
EMA geriatric medicines strategy. EMA. February 2011. https://www.ema.europa.eu/en/documents/other/geriatric-medicines-strategy_en.pdf. Accessed 09 February 2021.
Extermann M, Brain E, Canin B et al (2021) Priorities for the global advancement of care for older adults with cancer: an update of the international society of geriatric oncology priorities initiative. Lancet Oncol 22:29–36
Pérol D, Robain M, Arveux P et al (2019) The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the Epidemiological Strategy and Medical Economics (ESME). BMJ Open 9(2):e023568
Hamaker ME, Seynaeve C, Nortier JW et al (2013) Slow accrual of elderly patients with metastatic breast cancer in the Dutch multicenter OMEGA study. Breast 22(4):556–559. https://doi.org/10.1016/j.breast.2012.12.010
Sedrak MS, Mohile SG, Sun V et al (2020) Barriers to clinical trial enrollment of older adults with cancer: A qualitative study of the perceptions of community and academic oncologists. J Geriatr Oncol. 11(2):327–334
Lee JY, Lim SH, Lee MY et al (2016) The impacts of inclusion in clinical trials on outcomes among patients with metastatic breast cancer (MBC). PLoS ONE 11(2):e0149432. https://doi.org/10.1371/journal.pone.0149432
Hurria A, Dale W, Mooney M et al (2014) Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32(24):2587–2594. https://doi.org/10.1200/JCO.2013.55.0418
Sedrak MS, Freedman RA, Cohen HJ et al (2021) Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 71(1):78–92. https://doi.org/10.3322/caac.21638
Acknowledgements
The authors thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME contact for coordinating the project at the local level. We thank the ESME Scientific Committee members and the GERICO-DIALOG intergroup for their ongoing support.
Funding
This work was supported by R&D UNICANCER. The study was not funded in whole or in part by any research grant or funding body.
Author information
Authors and Affiliations
Contributions
MB conceived the study. MB, MC, AV, and CB contributed to the design of the study. MC contributed to the acquisition and analysis of the data. MB, MC, AV, and CB drafted the manuscript. All authors contributed to the interpretation of data, critically revised the manuscript, and gave final approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no potential conflict of interest relevant to the content of the manuscript.
Ethical approval
The ESME MBC database received approval from the French data protection authority (Commission Nationale de l’Informatique et des Libertés, authorisation no. 1704113).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bringuier, M., Carton, M., Levy, C. et al. Enrollment of older metastatic breast cancer patients in first-line clinical trials: 9-year experience of the large-scale real-life multicenter French ESME cohort. Breast Cancer Res Treat 191, 577–587 (2022). https://doi.org/10.1007/s10549-021-06467-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06467-2