Abstract
Purpose
Neurotensin receptor-1 (NTS1) is increasingly recognized as a potential target in diverse tumors including breast cancer, but factors associated with NTS1 expression have not been fully clarified.
Methods
We studied NTS1 expression using the Tissue MicroArray (TMA) of primary breast tumors from Institut Bergonié. We also studied association between NTS1 expression and clinical, pathological, and biological parameters, as well as patient outcomes.
Results
Out of 1419 primary breast tumors, moderate to strong positivity for NTS1 (≥ 10% of tumoral cells stained) was seen in 459 samples (32.4%). NTS1 staining was cytoplasmic in 304 tumors and nuclear in 155 tumors, a distribution which appeared mutually exclusive. Cytoplasmic overexpression of NTS1 was present in 21.5% of all breast tumors. In multivariate analysis, factors associated with cytoplasmic overexpression of NTS1 in breast cancer samples were higher tumor grade, Ki67 ≥ 20%, and higher pT stage. Cytoplasmic NTS1 was more frequent in tumors other than luminal A (30% versus 17.3%; p < 0.0001). Contrastingly, the main “correlates” of a nuclear location of NTS1 were estrogen receptor (ER) positivity, low E&E (Elston and Ellis) grade, Ki67 < 20%, and lower pT stage. In NTS1-positive samples, cytoplasmic expression of NTS1 was associated with shorter 10-year metastasis-free interval (p = 0.033) compared to NTS1 nuclear staining. Ancillary analysis showed NTS1 expression in 73% of invaded lymph nodes from NTS1-positive primaries.
Conclusion
NTS1 overexpression was found in about one-third of breast tumors from patients undergoing primary surgery with two distinct patterns of distribution, cytoplasmic distribution being more frequent in aggressive subtypes. These findings encourage the development of NTS1-targeting strategy, including radiopharmaceuticals for imaging and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The use of radiolabeled peptides to target tumor cells overexpressing peptide receptors for imaging and therapy is a promising and rapidly expanding approach. The use of the radiolabeled somatostatin analog 68Ga-DOTATATE and 177Lu- DOTATATE, respectively, for imaging and treatment of neuroendocrine tumors, is a well-known example, but many other (neuro)peptide receptors are overexpressed in diverse tumors or subsets of tumors and support the development of this personalized medicine [1]. The neurotensin receptor-1 (NTS1) is a G-protein-coupled receptor under investigation in several cancers. In cell lines, neurotensin (NT) exerts numerous oncogenic effects involved in tumor growth and metastatic spread [2,3,4]. Also, overexpression of NTS1 has been documented in many cancers and has been associated with higher grade tumors and a poorer outcome [2, 5,6,7]. In breast cancer, the work of Sandra Dupouy and co-workers gave initial insight on the potential role of the NT/NTS1-axis [8]. High NTS1 expression in invasive ductal carcinoma (IDC) was associated with larger tumor size and Scarff-Bloom-Richardson (SBR) grade 3. Also, breast cancer patients with high NTS1 expression had a significantly lower ten-year survival rate [8]. The same team focused on the association between NT, NTS1, and human epidermal growth factor receptors (HERs). Under NT stimulation, EGFR, HER2, and HER3 are up-regulated. NTS1-positive samples significantly overexpressed HER3, while no association was found between NTS1 and HER2 [9]. All together, these data support a role of NT/NTS1 to breast cancer progression. Identification of the subsets of breast cancer patients in whom NTS1 is likely to be expressed can lead the way to clinical applications of radiolabeled neurotensin analogs for imaging and therapy. In this work, we examined the expression of NTS1 within the fully-annotated Tissue MicroArray (TMA) of primary breast tumors from Institut Bergonié and studied association with clinical, pathological, and biological parameters and outcomes.
Materials and methods
The check-list of the reporting Recommendations for Tumor Marker Prognostic Study (REMARK) [10] is provided in supplemental file 1. Tissue MicroArray (TMA). We investigated the fully-annotated TMA of invasive breast cancers of Institut Bergonié, Bordeaux, France. Details on this TMA have been previously reported [11]. Briefly, samples in quadruplicates from 1419 invasive breast tumors from primary-operated patients between 2000 and 2005 who did not receive neoadjuvant chemotherapy or hormone therapy were available to study NTS1 expression.
Validation of the primary antibody raised against NTS1
As for IHC studies of other G-protein-coupled receptors, validation of the NTS1 antibody is of primary importance [12]. We followed the methodology we previously described for gastrin-releasing peptide receptor (GRP-R) [11]. Briefly, these steps included western blotting of breast cancer cell line lysates, immunochemistry of these cell lines fixed and embedded with the same material than that used for the construction of the TMA, identification of positive and negative tumors within a dedicated TMA, and validation of the signal recorded in IHC by western blotting of corresponding frozen tumors.
Immunochemistry
For IHC staining on TMA, 4-µm-thick paraffin-embedded tissue sections were mounted on slides. The whole IHC procedure was performed using the Ventana Benchmark-ultra automated staining system and according to the manufacturer’s recommendations. Antibody was diluted within a DAKO diluent (S2022) and unmasking was performed using a CC1 protocol at pH8. Antibody was incubated during 52 min at 1:50. Signal was detected using ultraVIEW Universal DAB (760–500). The primary antibody was a mouse monoclonal IgM kappa light chain (ref sc-376958, Santa Cruz Biotechnology, clone B12) raised against an epitope mapping between amino acids 389–417 at the C terminus of NTS1 of human origin, also used in other recent studies [13]. NTS1 expression using IHC was assessed by light microscopy by a senior pathologist blinded to the study endpoint who scored the location, the staining, its intensity, and the percentage of tumor cells stained. The mean number of tumoral cells stained for NTS1 for each TMA (in quadruplicates) core was reported.
Statistical analysis
For association with clinical, pathological, and biological parameters, NTS1 expression was dichotomized according to a cut-off of 10% stained cells, that has been previously suggested [2, 14, 15]. We studied associations between NTS1 expression and various clinical and pathological parameters. Differences between categorized variables were assessed with the χ2 test or Fischer exact test. All P values were two-sided and a p value of less than 0.05 was considered statistically significant. Factors that are predictive of high NTS1 expression were investigated by univariate and multivariate analyses using a logistic regression. Odds ratios and their 95% confidence intervals (95%CI) were reported. The median follow-up was calculated by the reverse Kaplan–Meier method. Probabilities of overall survival (OS) and distant metastasis-free interval (DMFI) were calculated with the Kaplan–Meier method. The logrank test was used for comparison between NTS1 groups. For OS, all causes of death were considered as events. For DMFI, all distant metastatic events were considered and patients with no metastases were censored at the date of last news. Multivariate analyses for DMFI were calculated with a Cox model. Variables significant at the 0.05 level in the univariate analysis were introduced in the multivariate model, which used a stepwise descending maximum likelihood method. Hazard ratio (HR) were calculated with a Cox model; proportionality was tested with the residuals test. For lymph node status, patients who did not have lymph node dissection (Nx) were kept in all analyses as a separate category. Statistical analyses were performed using the SAS software (version 9.3; SAS Institute, Inc.)
Lymph node metastases
In order to avoid bias due to unavailability of tissue for analysis, only samples from patients with 4 invaded nodes or more were included in this study. Thus, 14 lymph node metastases (one lymph node per patient), whose primary breast tumors were positive for NTS1, were considered for IHC in order to assess whether NTS1 expression is maintained in metastatic tissue. Three cases with nuclear staining were not analyzable (2 cases did not present enough biological material). Therefore analysis of NTS1 expression using IHC was run on 11 lymph node metastases samples from 11 primary tumors with high cytoplasmic NTS1 expression.
Results
Validation of the NTS1 primary antibody
The study of several human breast cancer cell lines showed a good correlation in NTS1 expression between western blot and immunocytochemistry assessment. Labeling was cytoplasmic and granular on both, immunofluorescence and immunochemistry (not shown). Moreover, the study of 26 tumor samples Holland-Bouin fixed and paraffin-embedded showed that the monoclonal antibody can significantly discriminate tumors that are positive for NTS1 from negative ones (p = 0.017), using as reference standard western blot analysis on frozen samples from the same tumors. All results are summarized in Fig. 1.
Validation of the NTS1 antibody. A Representative NTS1 band at the expected molecular weight of 55 kDa on western blot of frozen human tumors. B Representative actin band on western blot of frozen tumors. C The new monoclonal antibody is able to discriminate NTS1-positive tumors and NTS1-negative tumors. The IHC cut-off was ≥ 10% of cells stained
NTS1 expression in 1419 breast cancer samples, its cellular location, and association with pathological and biological parameters
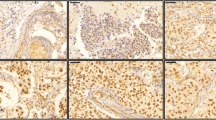
A moderate or high NTS1 expression (i.e., > 10% of tumor cells stained) was present in 32.4% of all breast cancer (459/1419) and exhibited a granular staining with either cytoplasmic location (n = 304; 21.5% of all tumors) or nuclear location (n = 155; 11.0% of all tumors) in cancerous cells (Fig. 2A, B).
A Representative NTS1 immunohistochemistry of a primary breast tumor sample from the tissue microarray with numerous cancerous cells exhibiting a cytoplasmic staining of NTS1 (magnification 40X). B Representative NTS1 immunohistochemistry of a primary breast tumor sample from the tissue microarray with numerous cancerous cells exhibiting a nuclear staining of NTS1 (magnification 40X)
Importantly, the nuclear vs cytoplasmic distribution appeared mutually exclusive. Only one case out of 459 NTS1-positive samples had both cytoplasmic and nuclear expression of NTS1. In the tumor-associated stromal tissue, mast cells were usually strongly positives for NTS1 [16]. Other cells of the tumor microenvironment showed no expression of NTS1.
In view of the dichotomy between cytoplasmic and nuclear location and because cytoplasmic location might be more easily amenable to targeting with radiopharmaceuticals, we carried out separate analysis of factors associated with NTS1 presence in breast cancer first at cytoplasmic level and then for nuclear localization.
Association between NTS1 positivity at cytoplasmic level and pathological and biological parameters in 1419 breast tumors
Cytoplasmic NTS1 staining was found in 304 tumors (21.5% of all tumors). In univariate analysis, factors associated with cytoplasmic NTS1 expression in tumors were a higher pT stage, a higher SBR grade, a node-positive status, a Ki67 score ≥ 20%, PR negativity, and positive HER2 status (Table 1). Age, histological subtype, and ER positivity level showed no significant association. The Low-Positive ER category in our TMA was defined prospectively as cancers with 1% to 9% of cells staining for ER; thus slightly different from the recent ASCO recommendation that put the upper limit for Low Positive as 10% [17]. In multivariate analysis, higher E&E grade, Ki67score ≥ 20%, and a higher pT stage remained significant predictors. On the other hand, breast cancer phenotype is strongly associated with cytoplasmic NTS1 expression (Table 1) but could not be introduced in the multivariate analysis because HER2, PR, and ER are part of the definition of the phenotypes. The luminal-A phenotype showed a lower probability of cytoplasmic NTS1 expression compared to the other phenotypes (17.3% for luminal A versus 32.9% for all other phenotypes combined; p < 0.0001).
Association between NTS1 positivity at nuclear level and pathological and biological parameters in 1419 breast tumors
Nuclear NTS1 staining was found in 155 tumors (11.0% of all tumors). In univariate analysis, factors associated with nuclear NTS1 expression were a lower pT stage, a lower E&E grade, a Ki67 score < 20%, a higher ER expression (≥ 10% vs. < 10% [combining negative and Low-Positive (1%–9%) categories], PR positivity, and absence of HER2 overexpression (Table 2). Age, histological subtype, and lymph node status showed no significant association. In multivariate analysis, higher ER level, lower pT stage, and lower E&E grade remained significant predictors. Breast cancer phenotype was strongly associated with nuclear NTS1 expression, with luminal-A phenotype showing the highest positivity rate, followed by luminal-B subtypes, while none of the ER-negative tumors expressed NTS1 at nuclear level (p < 0.0001) (Table 2).
Correlates of nuclear versus cytoplasmic location of NTS1
After exclusion of one case with mixed location, we performed an analysis on the 459 patients with NTS1-positive tumors in order to elucidate factors associated with nuclear (n = 155) versus cytoplasmic (n = 304) location of NTS1 (Table 3). There was no significant difference in age distribution or histological subtypes (invasive ductal/lobular). The subgroup of patients with nuclear NTS1 had, as compared to the subgroup with cytoplasmic NTS1: a lower rate of tumors > 2 cm (9.6% vs. 31.6%; p < 0.001), a lower rate of E&E grade III tumors (10.3% vs. 38.5%; p < 0.001), less tumors with Ki67 ≥ 20% (10.3% vs. 34.2%; p < 0.001), less patients with pathological-positive lymph nodes (27.7% vs. 39.5%; p = 0.019), less ER-negative tumors (0% vs. 11.8%; p < 0.00002), less PR-negative tumors (7.1% vs. 23.4%; p < 0.001), and less tumors with a HER2-positive status (3.9% vs. 14.8%; p < 0.001). As a result, when considering tumor phenotypes, the subgroup of patients with nuclear NTS1, as opposed to the subgroup with cytoplasmic NTS1, had higher rates of luminal-A tumors (83.2% vs. 53.9%), lower rates of luminal-B tumors regardless of HER2 status (16.8% vs. 34.2%) and no ER-negative subtypes (0% vs. 11.8%) (Table 3). Most strikingly, all tumors with nuclear NTS1 expression were ER-positive, with only 1/155 being ER Low Positive.
Association between cytoplasmic NTS1 expression and outcomes and between nuclear NTS1 expression and outcomes
Median follow-up was 122 months. Figure 2 shows Kaplan–Meier curves for DMFI and OS. When considering cytoplasmic NTS1 expression, there was no difference in OS (Logrank P = 0.2018) and only a trend toward lower DMFI (Logrank P = 0.053) for patients with cytoplasmic NTS1 expression compared to those without cytoplasmic NTS1 staining. Ten-year DMFI were 85.7% (95%CI: 80.8%–89.4%) and 89.5% (95%CI: 87.3%–91.3%), respectively. Considering nuclear NTS1, 10-year DMFI were 92.8% (95%CI: 86.9%–96.1%) for patients with nuclear NTS1 and 88.2% (95%CI: 86.1%–90.0%) for patients without nuclear NTS1 expression (Logrank P = 0.1145). There was no difference in OS (Logrank P = 0.6175).
Figure 3 shows DMFI and OS curves restricted to patients whose tumors expressed NTS1 and comparing outcomes of those with cytoplasmic location of NTS1 versus those with nuclear location of NTS1. There were no differences in OS (Logrank P = 0.2748). However a lower DMFI was found for patients with cytoplasmic NTS1 staining compared to those with nuclear NTS1 expression (Logrank P = 0.033). Patients with cytoplasmic expression of NTS1 had a twofold higher metastatic risk compared to patients with nuclear NTS1 staining (HR = 2.03; 95%CI: 1.04–3.94, p = 0.037). Differences in DMFI can be explained, at least in part, by the associations described above between cytoplasmic NTS1 expression and clinical and pathological characteristics. The number of events did not allow for performing a multivariable-adjusted Cox models. Further studies are needed to better discriminate any specific role of NTS1 on prognosis.
Distant metastasis-free interval according to the presence or absence of cytoplasmic A, or nuclear C NTS1 expression in primary breast tumors, or opposing patients with nuclear NTS1 expression to patients with cytoplasmic NTS1 expression (E). Association of cytoplasmic B, nuclear D, or nuclear vs cytoplasmic F NTS1 expression in primary tumors with OS
NTS1 expression in lymph node metastases from patients with NTS1-positive breast tumor
Invaded lymph nodes were available for the current study from 14 patients with NTS1-positive primary tumors. In three of these patients there was not enough residual material for IHC analysis. In all other 11 patients, NTS1 expression could be assessed on tumoral lymph node tissue. All 11 primary tumors had cytoplasmic NTS1 expression. In 8 (72.7%) of these cases, lymph node tumoral tissue also expressed NTS1 with cytoplasmic staining of NTS1 as shown in Fig. 4.
Discussion
The overexpression of neuropeptide receptors in breast cancer raises hope for the selective addressing of radiolabeled molecules [1, 18] or drugs using suitable cargos [19]. In this work we aimed to identify breast cancer patients who might benefit from NTS1-based imaging or targeted therapy.
We found NTS1 to be expressed in ~ 32% of breast tumors from patients who received primary surgery. In two-thirds of NTS1-positive cases, NTS1 showed cytoplasmic distribution, while in one-third of cases it was located in the nucleus. Interestingly, cytoplasmic versus nuclear location appeared mutually exclusive, with only one tumor showing mixed staining. Another important finding is that all 156 tumors with nuclear NTS1 staining were ER positive. Therefore, the sub-cellular location of NTS1 is strongly associated with estrogen receptor expression. Again, 92.9% of tumors with a nuclear expression of NTS1 expressed PR (Table 3). To further understand factors driving the sub-cellular location of NTS1, we performed an analysis on the 459 patients with NTS1-positive tumors. When compared to the subgroup with cytoplasmic NTS1 (n = 304), the subgroup of patients with nuclear NTS1 (n = 155) had lower rates of tumors > 2 cm, E&E grade III tumors, tumors with Ki-67 ≥ 20%, as well as less ER-negative tumors, PR-negative tumors ,and HER2-overexpressing tumors (Table 3).
It has been previously raised that neurotensin is expressed and up-regulated by estrogen in normal epithelial breast cells [8]. Also, women receiving high doses of steroids have increased immunoreactivity of neurotensin and NTS1 in human uterine myometrium and uterine leiomyomas [20]. On the other hand, neurotensin regulates the expression of NTS1 in different cell compartments [20,21,22]. Under activation by neurotensin, NTS1 is internalized to the perinuclear region [21] or addressed to the nuclear envelope and the nucleus soma [22] due to the strong nuclear-targeting signal contained in the NTS1 receptor [23]. The NTS2 receptor subtype may also participate in the intracellular processing of NTS1. It has been suggested that heterodimerization of NTS1 with NTS2 may cause the retention of NTS1 in sub-cellular compartments and interfere with its proper recruitment to the plasma membrane [24]. These mechanisms of autocrine/paracrine activating loops deserve further investigations but may be tumor specific as, we and others, did not find nuclear localization of NTS1 in primary prostate tumors [15, 25], whereas a nuclear localization of NTS1 was found in gastro-intestinal stromal tumors [26]. Nuclear localization of other G-protein-coupled receptors has been described [27]. Whether the nuclear trafficking of NTS1 receptor would allow transporting NTS1-targeting agents to the nucleus deserves investigation [28,29,30].
Cytoplasmic NTS1 location facilitates targeting with radiopharmaceuticals. It is of interest that cytoplasmic overexpression of NTS1 was found more frequently in tumors with poorer prognostic factors (E&E grade II/III, Ki67 ≥ 20%, HER2 positive status, ER negativity, and PR negativity). Similar results regarding cytoplasmic expression of NTS1 in breast cancer were found in earlier studies [8, 9]. As regarding breast cancer phenotypes, our study further shows that the probability of cytoplasmic NTS1 expression is higher in higher-risk phenotypes. Cytoplasmic expression of NTS1 is found in 17.3% in luminal-A tumors and in ~ 30% of the other phenotypes combined (p < 0.0001). Another important finding for therapeutic purposes is that NTS1 expression is most often preserved in nodal metastases from NTS1-positive primary tumors (8/11: 72.7%) (Fig. 3), but this observation requires confirmation, and studies of NTS1 expression in distant metastases are needed. Our results support recent findings on the genomic data of NTS1 mRNA in primary breast tumors with axillary lymph node metastasis [31]. Therefore, in N+ breast cancer patients with luminal-B phenotype, or HER2-enriched phenotype, or triple-negative phenotype, the development of NTS1-targeted agents would be of interest for imaging and/or therapy of these subsets of patients.
Dupouy and co-workers found that breast cancer patients with cytoplasmic overexpression of NTS1 had a higher relative risk of dying [8]. In our series of mostly low risk (primary operable breast cancer) we found no difference in OS, but a lower DMFI (Logrank P = 0.033) for patients with cytoplasmic NTS1 expression compared to those with nuclear NTS1 staining. 10-year DMFI were 85.7% and 92.8%, respectively.
A pilot study was performed in 6 patients with metastatic pancreatic adenocarcinoma, patients using low doses (1.5 GBq) of the radiolabeled NTS1 antagonist [177Lu]Lu-3BP-227 for imaging, followed by higher therapeutic activities in patients with adequate tumor uptake on imaging [32]. This pilot study shows the current interest in NTS1 targeting and many more NTS1-targeting radiopharmaceuticals for imaging and therapy is being developed [33,34,35]. In breast cancer, NTS1 imaging using Positron Emission Tomography (PET) or Single Photon Emission Computed Tomography (SPECT) would be beneficial in specific phenotypes. In triple-negative breast cancer, identification of NTS1 as a potentially targetable receptor paves the way of an “image and treat” strategy using 68 Ga- and 177Lu-labeled radiopharmaceuticals. Interestingly, Dupouy and colleague, reported that neurotensin/NTS1 promotes activation of EGFR, HER2, and HER3 rendering breast tumors aggressive yet highly responsive to lapatinib and metformin in mice [9] and NTS1 imaging might also be interesting in this regard. Finally, exploring tumor heterogeneity by both [18F]-FDG PET [36] and NTS1-PET might offer complementary staging and prognostic information and would be beneficial to better predict response to targeted radionuclide therapy as today performed in neuroendocrine tumors (using [68Ga]Ga-DOTATATE/[18F]-FDG) [37] and prostate cancer (using [68Ga]Ga-PSMA/[18F]-FDG) [38].
Our study has limitations inherent to the use of a TMA. We could only assess the NTS1 expression in the peritumoral environment of the TMA cores which may reflect only partially the expression of NTS1 by normal breast cells. Moreover, the low number of samples is too small to draw solid conclusion as compared to the NTS1 expression in related metastatic lymph nodes. Finally, future studies of NTS1 expression in distant metastases will also be needed to consolidate the “theranostic” potential of this new target in breast cancer.
As perspectives and before considering future NTS1-targeted therapeutic applications, assessing the effect of previous treatments (hormone therapy or chemotherapy) on NTS1 expression is a necessary step. This work is scheduled in our consortium.
Conclusion
Our data from this large tissue bank documents the overexpression of NTS1 in about one-third of primary breast tumors with a dichotomic intracellular distribution (cytoplasmic vs. nuclear). Cytoplasmic overexpression of NTS1 was found in 21.5% of breast tumors from patients undergoing primary surgery, with higher frequency in aggressive subtypes. These findings encourage the development of radiopharmaceuticals for imaging and therapy. On the other hand, nuclear NTS1 location, found in 11% of patient samples, and its strong association with ER positivity deserve investigation of the mechanisms involved. We hope that the expression profile we described in this study would stimulate new developments for imaging and therapy of breast cancer patients using NTS1-targeted agents.
References
Morgat C, Mishra AK, Varshney R et al (2014) Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. J Nucl Med 55:1650–1657. https://doi.org/10.2967/jnumed.114.142000
Younes M, Wu Z, Dupouy S et al (2014) Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 5:8252–8269
Ye Y, Long X, Zhang L et al (2016) NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 7:70303–70322. https://doi.org/10.18632/oncotarget.11854
Zhang Y, Zhu S, Yi L et al (2014) Neurotensin receptor1 antagonist SR48692 reduces proliferation by inducing apoptosis and cell cycle arrest in melanoma cells. Mol Cell Biochem 389:1–8. https://doi.org/10.1007/s11010-013-1920-3
Körner M, Waser B, Strobel O et al (2015) Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. https://doi.org/10.1186/s13550-015-0094-2
Agopiantz M, Forgez P, Casse J-M et al (2017) Expression of neurotensin receptor 1 in endometrial adenocarcinoma is correlated with histological grade and clinical outcome. Virchows Arch 471:521–530. https://doi.org/10.1007/s00428-017-2215-y
Zhou Z, Zhou Z, Xie J et al (2015) The significance of NTR1 expression and its correlation with β-catenin and EGFR in gastric cancer. Diagn Pathol 10:128. https://doi.org/10.1186/s13000-015-0356-3
Dupouy S, Viardot-Foucault V, Alifano M et al (2009) The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS One 4:e4223. https://doi.org/10.1371/journal.pone.0004223
Dupouy S, Doan VK, Wu Z et al (2014) Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget 5:8235–8251
Sauerbrei W, Taube SE, McShane LM et al (2018) Reporting Recommendations for tumor marker prognostic studies (REMARK): An abridged explanation and elaboration. J Natl Cancer Inst 110:803–811. https://doi.org/10.1093/jnci/djy088
Morgat C, Macgrogan G, Brouste V et al (2017) Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: A study of 1,432 primary tumors. J Nucl Med 58:1401–1407
Reubi JC (2014) Strict rules are needed for validation of G-protein-coupled receptor immunohistochemical studies in human tissues. Endocrine 47:659–661. https://doi.org/10.1007/s12020-014-0320-0
Riehle KJ, Kenerson HL, Riggle KM et al (2019) Neurotensin as a source of cyclic AMP and co-mitogen in fibrolamellar hepatocellular carcinoma. Oncotarget 10:5092–5102. https://doi.org/10.18632/oncotarget.27149
Souazé F, Dupouy S, Viardot-Foucault V et al (2006) Expression of neurotensin and NT1 receptor in human breast cancer: A potential role in tumor progression. Cancer Res 66:6243–6249. https://doi.org/10.1158/0008-5472.CAN-06-0450
Morgat C, Chastel A, Molinie V et al (2019) Neurotensin receptor-1 expression in human prostate cancer: A pilot study on primary tumors and lymph node metastases. Int J Mol Sci 20:1721. https://doi.org/10.3390/ijms20071721
Cochrane DE, Carraway RE, Harrington K et al (2011) HMC-1 human mast cells synthesize neurotensin (NT) precursor, secrete bioactive NT-like peptide(s) and express NT receptor NTS1. Inflamm Res 60:1139–1151. https://doi.org/10.1007/s00011-011-0378-6
Allison KH, Hammond MEH, Dowsett M et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. https://doi.org/10.1200/JCO.19.02309
Chastel A, Worm DJ, Alves ID et al (2020) Design, synthesis, and biological evaluation of a multifunctional neuropeptide-Y conjugate for selective nuclear delivery of radiolanthanides. EJNMMI Res 10:16. https://doi.org/10.1186/s13550-020-0612-8
Bird JL, Simpson R, Vllasaliu D, Goddard AD (2017) Neurotensin receptor 1 facilitates intracellular and transepithelial delivery of macromolecules. Eur J Pharm Biopharm 119:300–309. https://doi.org/10.1016/j.ejpb.2017.06.027
Rodríguez Y, Almeida TA, Valladares F et al (2010) Neurotensin and neurotensin receptor 1 expression in human myometrium and uterine leiomyomas. Biol Reprod 83:641–647. https://doi.org/10.1095/biolreprod.110.084962
Toy-Miou-Leong M, Cortes CL, Beaudet A et al (2004) Receptor trafficking via the perinuclear recycling compartment accompanied by cell division is necessary for permanent neurotensin cell sensitization and leads to chronic mitogen-activated protein kinase activation. J Biol Chem 279:12636–12646. https://doi.org/10.1074/jbc.M303384200
Toy-Miou-Leong M, Bachelet C-M, Pélaprat D et al (2004) NT agonist regulates expression of nuclear high-affinity neurotensin receptors. J Histochem Cytochem 52:335–345. https://doi.org/10.1177/002215540405200304
Feldberg RS, Cochrane DE, Carraway RE et al (1998) Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm res 47:245–250. https://doi.org/10.1007/s000110050325
Hwang JR, Baek MW, Sim J et al (2010) Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem Biophys Res Commun 391:1007–1013. https://doi.org/10.1016/j.bbrc.2009.12.007
He T, Wang M, Wang H et al (2019) Evaluation of neurotensin receptor 1 as potential biomarker for prostate cancer theranostic use. Eur J Nucl Med Mol Imaging 46:2199–2207. https://doi.org/10.1007/s00259-019-04355-y
Gromova P, Rubin BP, Thys A et al (2011) Neurotensin receptor 1 Is expressed in gastrointestinal stromal tumors but not in interstitial cells of cajal. PLoS One 6:e14710. https://doi.org/10.1371/journal.pone.0014710
Zhang X, Fan S, Zhang L, Shi Y (2020) Glucagon-like peptide-1 receptor undergoes importin-α-dependent nuclear localization in rat aortic smooth muscle cells. FEBS Lett 594:1506–1516. https://doi.org/10.1002/1873-3468.13751
Müller C, Umbricht CA, Gracheva N et al (2019) Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04345-0
Hindié E, Zanotti-Fregonara P, Quinto MA et al (2016) Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various Sizes: implications for radiopharmaceutical therapy. J Nucl Med 57:759–764
Alcocer-Ávila ME, Ferreira A, Quinto MA et al (2020) Radiation doses from 161Tb and 177Lu in single tumour cells and micrometastases. EJNMMI Physics 7:33. https://doi.org/10.1186/s40658-020-00301-2
Callegari CCF, Cavalli IJ, Lima RS et al (2016) Copy number and expression analysis of FOSL1, GSTP1, NTSR1, FADD and CCND1 genes in primary breast tumors with axillary lymph node metastasis. Cancer Genet 209:331–339. https://doi.org/10.1016/j.cancergen.2016.06.003
Baum RP, Singh A, Schuchardt C et al (2018) 177Lu-3BP-227 for neurotensin receptor 1–targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J Nucl Med 59:809–814. https://doi.org/10.2967/jnumed.117.193847
Maschauer S, Einsiedel J, Hübner H et al (2016) 18F- and 68Ga-labeled neurotensin peptides for PET imaging of neurotensin receptor 1. J Med Chem 59:6480–6492. https://doi.org/10.1021/acs.jmedchem.6b00675
Feng H, Zhang H, Wang M et al (2020) Improving tumor-to-background contrast through hydrophilic tetrazines: The construction of 18f labeled pet agents targeting non-small cell lung carcinoma. Chemistry 26:4690–4694. https://doi.org/10.1002/chem.202000028
Fanelli R, Chastel A, Previti S et al (2020) Silicon-containing neurotensin analogues as radiopharmaceuticals for NTS1-positive tumors imaging. Bioconjugate Chem 31:2339–2349. https://doi.org/10.1021/acs.bioconjchem.0c00419
Groheux D, Hindie E (2021) Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging 9:221–231. https://doi.org/10.1007/s40336-021-00426-z
Chan DL, Pavlakis N, Schembri GP et al (2017) Dual somatostatin Receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics 7:1149–1158. https://doi.org/10.7150/thno.18068
Hofman MS, Emmett L, Sandhu S et al (2021) [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397:797–804. https://doi.org/10.1016/S0140-6736(21)00237-3
Funding
This work was funded by Institut National du Cancer (INCa PLBIO 2017, THERACAN project) and was achieved within the context of the Laboratory of Excellence TRAIL ANR-10-LABX-57.
Author information
Authors and Affiliations
Contributions
CM contributes to the conception, acquisition, analysis, interpretation of data, and funding and wrote the manuscript. VB analyzed and interpreted the data and approved the final version of the manuscript. AC contributes to data acquisition and approved the final version of the manuscript. VV acquired the data and approved the final version of the manuscript. GMG acquired and analyzed the data and approved the final version of the manuscript. EH analyzed and interpreted the data, participated in the funding, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Institut Bergonié (Breast Research Group) who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRB of Institut Bergonié.
Informed consent
The Ethics committee of Institut Bergonié waived requirement for informed consent for the present retrospective analysis.
Cell lines
MCF-7, MDA-MB-453, MDA-MB-468, SKBR3, T47D, and ZR75.1 cell lines were obtained from Dr N. Jones (Univ. Bordeaux, France) and no additional authentication was performed by the authors of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morgat, C., Brouste, V., Chastel, A. et al. Expression of neurotensin receptor-1 (NTS1) in primary breast tumors, cellular distribution, and association with clinical and biological factors. Breast Cancer Res Treat 190, 403–413 (2021). https://doi.org/10.1007/s10549-021-06402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06402-5