Abstract
Purpose
We examined the associations of mammographic breast density with breast cancer risk by tumor aggressiveness and by menopausal status and current postmenopausal hormone therapy.
Methods
This study included 2596 invasive breast cancer cases and 4059 controls selected from participants of four nested case–control studies within four established cohorts: the Mayo Mammography Health Study, the Nurses’ Health Study, Nurses’ Health Study II, and San Francisco Mammography Registry. Percent breast density (PD), absolute dense (DA), and non-dense areas (NDA) were assessed from digitized film-screen mammograms using a computer-assisted threshold technique and standardized across studies. We used polytomous logistic regression to quantify the associations of breast density with breast cancer risk by tumor aggressiveness (defined as presence of at least two of the following tumor characteristics: size ≥2 cm, grade 2/3, ER-negative status, or positive nodes), stratified by menopausal status and current hormone therapy.
Results
Overall, the positive association of PD and borderline inverse association of NDA with breast cancer risk was stronger in aggressive vs. non-aggressive tumors (≥51 vs. 11–25% OR 2.50, 95% CI 1.94–3.22 vs. OR 2.03, 95% CI 1.70–2.43, p-heterogeneity = 0.03; NDA 4th vs. 2nd quartile OR 0.54, 95% CI 0.41–0.70 vs. OR 0.71, 95% CI 0.59–0.85, p-heterogeneity = 0.07). However, there were no differences in the association of DA with breast cancer by aggressive status. In the stratified analysis, there was also evidence of a stronger association of PD and NDA with aggressive tumors among postmenopausal women and, in particular, current estrogen+progesterone users (≥51 vs. 11–25% OR 3.24, 95% CI 1.75–6.00 vs. OR 1.93, 95% CI 1.25–2.98, p-heterogeneity = 0.01; NDA 4th vs. 2nd quartile OR 0.43, 95% CI 0.21–0.85 vs. OR 0.56, 95% CI 0.35–0.89, p-heterogeneity = 0.01), even though the interaction was not significant.
Conclusion
Our findings suggest that associations of mammographic density with breast cancer risk differ by tumor aggressiveness. While there was no strong evidence that these associations differed by menopausal status or hormone therapy, they did appear more prominent among current estrogen+progesterone users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammographic breast density is a well-established and strong predictor of breast cancer risk [1,2,3,4]. Women with breasts of 75% or greater percent density (proportion of the total breast area that appears dense on the mammogram) are at 3- to 5-fold greater risk of breast cancer compared to women with mostly fatty breasts [3, 5, 6]. Absolute dense area of the breast that represents amount of fibroglandular tissue has also been shown to be positively associated with breast cancer risk [7,8,9,10,11,12,13], while non-dense area of the breast (representing adipose tissue) is inversely associated with breast cancer risk [7, 14].
A recent study found a stronger association of breast density defined with Breast Imaging Reporting and Data System classification (American College of Radiology) with breast cancer in premenopausal women and postmenopausal hormone users compared with postmenopausal women not on hormones [15]. We found similar results in an analysis from the Nurses’ Health Study: the association between percent density and breast cancer risk appeared to be stronger in premenopausal women than in postmenopausal women without postmenopausal hormone use history and among postmenopausal women currently using hormones compared to postmenopausal women who never used hormones or with past hormone use [16]. Our previous studies have also demonstrated stronger associations between breast density and breast cancer subtypes with individual aggressive tumor characteristics including larger size, higher grade, estrogen receptor (ER) negative status, and positive nodal involvement [17, 18]. One study to date has shown increased risk of advanced stage breast cancer for postmenopausal hormone therapy users who had very high density (BI-RADS 4) compared to those with average density (BI-RADS 2) [15]. We extend this prior work by examining the associations of quantitative measures of breast density with tumor aggressiveness, defined using combination of aggressive tumor features such as higher grade, larger size, ER-negative status, and nodal involvement rather than individual tumor features. These features have been consistently linked to more aggressive tumor behavior and poorer survival [19,20,21,22,23,24,25]; tumor size and nodal involvement are used for breast cancer clinical staging [26]. We further examine these associations by menopausal status and postmenopausal hormone use. We used the data from four prospective cohorts to examine if associations of breast density phenotypes (percent breast density, absolute dense area, and non-dense area) with breast cancer risk differ by tumor aggressiveness, menopausal status, and current hormone use.
Methods
Study populations
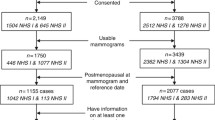
Women included in this study were selected from participants of the Mayo Mammography Health Study (MMHS), the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHSII), and San Francisco Mammography Registry (SFMR). These cohorts have been previously described in detail elsewhere [27,28,29,30,31,32,33,34]. From each cohort, a nested case–control study contributed participants to the current analysis.
MMHS is a prospective mammography cohort that recruited women from Minnesota, Wisconsin, and Iowa who were at age 35 years or older and had a screening mammogram at the Mayo Clinic during 2003–2006. Women without breast cancer (controls) were matched to incident breast cancer cases on age, menopausal status, year of exam, and state. This cohort contributed 372 cases and 638 controls to the current analysis.
NHS and NHSII are prospective cohorts that followed registered nurses in the United States who were 30–55 years (NHS) or 25–42 years old (NHSII) at enrollment. Breast cancer cases were confirmed through medical record review by trained personnel. A nested case–control study within these cohorts was previously established to examine associations of various circulating biomarkers with breast cancer risk [17]. Women without cancer history (other than non-melanoma skin cancer) were matched 1:1 or 1:2 with breast cancer cases on age at the time of blood collection, menopausal status, and postmenopausal hormone use (current vs. not current) at blood draw, and day/time of blood draw; for NHS II, additional matching included race/ethnicity and day in the luteal phase [35]. This study contributed 912 cases and 1109 controls to the current analysis.
The SFMR is a population-based registry that collects demographic, clinical and risk factor information, mammographic findings, and cancer outcomes through linkage with state-wide California SEER program. A nested case–control study within this cohort contributed 1312 cases and 2312 controls to the current analysis. Cases were matched to controls on age, menopausal status, and year of mammogram.
In total, this analysis included 2596 invasive breast cancer cases and 4059 controls within these cohorts. This study was approved by the Institutional Review Boards at the Mayo Clinic, Brigham and Women’s Hospital, and the University of California, San Francisco. Informed consent was obtained or implied by return of questionnaires (NHS, NHSII).
From all studies, we excluded breast cancer cases diagnosed within 6 months of mammography and their matched controls, to minimize prevalent cancers at the time of mammography.
Assessment of mammographic breast density
Mammographic breast density was estimated on digitized pre-diagnostic film-screen mammograms of the craniocaudal view using computer-assisted threshold techniques (Cumulus [36] and UCSF custom mammographic density software [37]). The two methods have previously shown very high agreement (intraclass correlation 0.94) [37]. Percent breast density was measured as percentage of the total area occupied by epithelial/stromal tissue (absolute dense area) divided by the total breast area. For NHS and NHSII mammograms, the average density of both breasts was used in the analysis. For MMHS and SFMR, breast density was estimated from the contralateral breast for cases and the corresponding side for matched controls. As reported previously, densities of the right and left breast for an individual woman are strongly correlated (Pearson correlation coefficient 0.86–0.96) [38] and the average density from both breasts is similar to density assessed on a randomly selected side [39].
Percent breast density, absolute dense area, and non-dense area measures were standardized across studies to account for inter-rater variability in the density assessment as previously described [18].
Assessment of breast tumor aggressiveness
Information on tumor type, histology, grade, nodal involvement, and tumor size was obtained from state-wide Surveillance Epidemiology and End Results programs (SFMR), pathology reports (NHS and NHSII), and state and clinic cancer registries and medical records (MMHS). Recent studies demonstrate that use of histomorphological characteristics of the tumor improves the prognostic accuracy of breast cancer staging [40]. In our analysis, tumors were defined as aggressive if they had at least two of the following criteria: size 2 cm and greater, differentiation grade 2 or 3, ER-negative status, and positive nodes. These histomorphological characteristics have been previously linked to poorer prognosis and patient survival [23,24,25, 41,42,43,44]. This approach takes into account both clinical characteristics (nodal involvement and tumor size) as well as selected histological and molecular features such as grade and ER status, respectively. Cases with unknown size, grade, nodal status, or ER status were excluded from the analysis (n = 659 or 20.2%). Characteristics of the cases included in the analysis were similar to characteristics of the cases excluded from this study due to the missing tumor characteristics (data not shown).
Covariates
Covariate information was obtained from self-administered questionnaires prior to mammography (NHS, NHSII, SFMR) or both self-administered questionnaires and medical record review at the time of mammography (MMHS). Cases with unknown menopausal status and postmenopausal women with unknown hormone therapy status were excluded from analysis (n = 490 or 15.7%). Control women who were previously matched to eligible cases were included in analyses unless they had unknown menopausal or hormone therapy status (n = 470 or 10.4% excluded). Characteristics of the women included in the analysis were similar to characteristics of those excluded from this study due to the missing data on menopausal status and hormone therapy, except for age, menopausal status, and hormone therapy as expected based on the exclusion criteria.
Statistical analysis
Standardized percent breast density was categorized as 0–10, 11–25% (reference), 26–50, and ≥51%, consistent with the previous analyses [18, 34, 45]. Absolute dense and non-dense areas were defined as quartiles based on the distribution in controls (absolute dense area: 1st: 0.0–20.0; 2nd: 20.1–36.6; 3rd: 36.7–60.5; 4th: >60.6 cm2; non-dense area: 1st: 4.2–70.3; 2nd: 70.4–117.2; 3rd: 117.3–188.9; 4th: >189.0 cm2). We used polytomous logistic regression to describe the associations of breast density measures with breast cancer risk by tumor aggressiveness, overall and stratified by woman’s menopausal status and current hormone therapy (premenopausal, postmenopausal/estrogen therapy alone, postmenopausal/combined estrogen+progesterone therapy, and postmenopausal/no hormones). In this pooled analyses from four studies, the risk estimates were adjusted for study site, age (continuous), and body mass index (continuous). We further considered potential confounders including parity (nulliparous, parous, or unknown) and first-degree family history of breast cancer (yes, no, or unknown) by evaluating the magnitude of the change in odds ratios (OR) observed after including each potential confounder individually in the model. Addition of these variables to the models did not substantially change risk estimates and they were not included in the final models.
We first evaluated whether the associations of breast density measures with breast cancer risk differed for aggressive vs. non-aggressive tumor subtypes. Contrasts were used to construct a test of association of density by aggressiveness (p-heterogeneity) within the polytomous regression framework to investigate whether there was statistical evidence of differences in association of density with breast cancer risk by tumor aggressiveness. For these heterogeneity tests, density was modeled using an ordinal trend across quartiles in order to increase power. We next examined tests of two-way interactions to assess the significance of the differences in associations of breast density measures with tumor aggressiveness across the strata defined by woman’s menopausal status and current hormone use using Wald Chi-square test. Finally, contrasts were also used to assess heterogeneity of the risk estimates by tumor aggressiveness within each of the strata.
We assessed the statistical significance of differences in associations by study through testing for interactions between study group and density in the pooled analysis, and found no evidence of differences across the studies (p-heterogeneity for percent density = 0.78, for absolute dense area = 0.26, and for non-dense area = 0.54).
In a secondary analysis, we excluded cases with mammogram date within 2 years of diagnosis (15% of the cases). Analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). For all analyses, the level of statistical significance was assessed at 0.05 level. All tests were two-sided.
Results
Distribution of breast cancer risk factors by menopausal status and hormone use among cases and controls is presented in Table 1. Compared to controls, cases had a greater percent density and a larger absolute dense area in all strata. Cases also had a smaller area of non-dense breast tissue in all strata except postmenopausal women with no hormone use. There were no differences in BMI by case and control status among premenopausal women. Among postmenopausal women, mean BMI was greater among cases vs. controls across all strata but only reached statistical significance among those with no hormone use (27.3 vs. 26.1 kg/cm2, p < 0.001). Further, there was a larger proportion of cases than controls with a positive family history of breast cancer, although only associations among the two largest groups, premenopausal and postmenopausal women not using hormones, were statistically significant (p < 0.001 for both).
Distribution of tumor grade, size, ER status, and nodal involvement by tumor aggressiveness are presented in Supplementary Table 1. Among cases defined as aggressive tumors, 52 (7%) met all four criteria, 219 (29%) met three criteria, and 473 (64%) cases met two of the four criteria. Among aggressive tumors, 46.6% were estrogen receptor-negative as compared to 4.2% among non-aggressive tumors.
In the overall analysis, percent breast density was more strongly associated with the risk of aggressive breast tumor subtypes vs. non-aggressive tumors (OR 2.50, 95% CI 1.94–3.22 vs. OR 2.03, 95% CI 1.70–2.43 for density ≥51 vs. 11–25%, respectively, p-heterogeneity = 0.03). The inverse association of non-dense area with breast cancer risk appeared to be stronger in aggressive breast tumor subtypes vs. non-aggressive tumors, though the difference did not reach statistical significance (OR 0.54, 95% CI 0.41–0.70 vs. OR 0.71, 95% CI 0.59–0.85 for 4th vs. 2nd quartile, respectively, p-heterogeneity = 0.07). The association of absolute dense area with the risk of breast cancer was similar for aggressive and non-aggressive tumors (OR 1.74, 95% CI 1.40–2.17 vs. OR 1.72, 95% CI 1.47–2.00 for 4th vs. 2nd quartile, respectively, p-heterogeneity = 0.45) (Table 2). We found no differences in the associations of breast density phenotypes with tumor aggressiveness by menopausal status/hormone therapy with any of the density measures (p-interaction = 0.80 for percent density, 0.91 for absolute dense area, and 0.36 for non-dense area) (Table 3). However, the largest significant difference in the risk estimates for aggressive vs. non-aggressive subtypes was noted for percent density and non-dense area among postmenopausal women, and in particular among those with combined estrogen+progesterone therapy (OR 3.24 vs. OR 1.93, respectively for density ≥51 vs. 11–25%; p-heterogeneity = 0.01; OR 0.43 vs. 0.56, respectively for 4th vs. 2nd quartile; p-heterogeneity = 0.01).
In a secondary analysis excluding cases with mammogram date within 2 years of diagnosis the results were similar (Supplementary Table 2).
Discussion
In this pooled analysis of four nested case–control studies, we investigated the association of breast density phenotypes with breast cancer risk according to tumor aggressiveness among 2596 women who developed breast cancer and 4059 matched controls. A stronger positive association of percent density and inverse association of non-dense area with aggressiveness of breast cancer were noted in the overall analysis. While there was no strong evidence that these associations differed by menopausal status or hormone therapy, they did appear more prominent among current estrogen and progesterone users.
We examine the associations of breast density phenotypes with the risk of breast cancer by tumor’s aggressiveness defined using combination of histomorphological characteristics reflective of aggressive tumor behavior. Previous studies on the association of breast density with breast cancer subtypes, including our own [17, 18], examined these characteristics individually. The tumor represents a combination of histomorphological features and these features do not exist in isolation from one another. Recent studies demonstrate that use of histomorphological characteristics of the tumor improves the prognostic accuracy of breast cancer staging [40]. We could not, however, incorporate PR and HER2 receptor statuses in this classification as it resulted in significant decrease in sample size and loss in statistical power, especially since the primary aim of our analysis was to investigate differences in associations by menopausal status and hormone use.
Positive associations of breast density with tumor size, involvement of axillary nodes, and higher tumor grade have been reported in some [46,47,48], but not all studies [49]. We recently reported stronger associations of percent density with ER− tumors vs. ER+ tumors, as well as for larger tumors and tumors with higher grade in postmenopausal women from NHS [17]. In a pooled analysis which included the NHS, we found a stronger association of percent density with larger tumors and positive nodal involvement, but the stronger associations with ER− tumors were limited to women aged <55 years [18]. The results from our current analysis indicate a stronger association of percent density and suggestive stronger inverse association of non-dense area with more aggressive subtypes of breast cancer, and this association did not vary by menopausal status or hormone therapy. If breast density is associated with more aggressive breast cancer phenotypes it may suggest that the breast tissue environment underlying high breast density allows for more growth and increased proliferation, than in more fatty breasts. In addition, since mammographic sensitivity decreases with increasing density, aggressive cancers occurring in denser breasts may go undetected for a longer period of time permitting these already rapidly proliferating tumors to be larger at presentation [50,51,52,53]. A recent study by Kerlikowske et al. [15] found a stronger association of BI-RADS-defined breast density with the risk of more advanced stage of disease, mostly among postmenopausal women taking estrogen and progesterone hormone therapy, consistent with our suggestive findings in this study subgroup. In contrast to our study, this study used the TNM system based on the criteria of the American Joint Committee on Cancer to define advance disease stage (stage I or IIA: early-stage invasive cancer; IIB, III, or IV: late stage invasive cancer) [54]. This classification, however, does not take into account tumor grade and ER status, important characteristics reflective of tumor aggressiveness that has been consistently linked to less favorable tumor subtypes and worse patient outcomes [19,20,21,22,23,24,25]. Tumor grade has been incorporated in prognostic algorithms for treatment decision making [55] and has been shown to correlate with molecular signatures of the tumor (genetic, transcriptomic, and microarray-based genomic) [56,57,58,59]. Our current approach for classification of tumor subtypes accounts for various tumor features using their combination rather than individual characteristics and incorporates important information on tumor grade.
We found no evidence of interaction between menopausal status/current hormone use and breast density on tumor aggressiveness in the overall analysis. Kerlikowske and colleagues [15] found a stronger association of high breast density estimated by BI-RADS with breast cancer in premenopausal women and postmenopausal hormone users compared with postmenopausal women not on hormones. The study included invasive and in situ breast cancers identified among participants from seven mammography registries that participate in the Breast Cancer Surveillance Consortium [15]. The comparison of associations between breast density and tumor stage by menopausal status and postmenopausal hormone use included 10,514 invasive breast cancer cases with complete information on important study variables. The analysis in this study, however, was not stratified by the type of the current HT. The differences in our findings may be due to limited power in the current analysis. In our recent analysis from Nurses’ Health Study, which is included in the sample here, the association between percent density and breast cancer risk appeared to be stronger in premenopausal women than in postmenopausal women without postmenopausal hormone use history and appeared to be stronger in postmenopausal women currently using hormones compared to postmenopausal women who never used postmenopausal hormones or with past hormone use [16]. That study, however, did not examine different regimens of current therapy use and our current analysis did not include a separate category for past hormone users, limiting our comparison.
Strengths of this study include data from four established prospective cohorts with comprehensive follow-up, breast cancer risk factors, tumor characteristics, and standardized breast density estimates. Use of matching in the four nested case–control studies utilized in the current investigation allows us to minimize the potential confounding effect of selected factors. This study also has a few limitations. We pooled data from four different studies, which varied in study design, population characteristics, geography, and calendar year. Our study used clinical pathology information and not centralized pathology review. However, we found no evidence of between-study heterogeneity in our results and all analyses were adjusted for the study site. The study population was predominantly white which might reduce generalizability of the findings to non-Caucasian populations. We excluded women who did not have information on tumor characteristics, menopausal status, or hormone use but these were not materially different from those included in analyses. Finally, insufficient data on the tumor detection method (screening-detected vs. interval-detected cancer) did not allow us to clarify the extent to which our observations reflect delays in diagnosis rather than tumor biology.
In conclusion, we investigated the associations of mammographic breast density with breast cancer risk by combination of tumor’s aggressiveness features and woman’s menopausal status/current hormone therapy. Our results suggest that percent mammographic breast density is positively associated with the tumor aggressiveness while non-dense area is potentially inversely associated with the aggressiveness of tumors. These differences may be more prominent among postmenopausal women currently using estrogen and progesterone. Further studies are warranted to explain underlying biological processes and elucidate the possible pathways from high breast density to the aggressive subtypes of breast carcinomas. If our results are confirmed in subsequent investigations, the findings would suggest that early detection in this subgroup might be especially important to optimize breast cancer survival.
References
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808
Ginsburg OM, Martin LJ, Boyd NF (2008) Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer 99(9):1369–1374
Tamimi RM, Byrne C, Colditz GA, Hankinson SE (2007) Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 99(15):1178–1187
Harvey JA, Bovbjerg VE (2004) Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology 230(1):29–41
Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 87(9):670–675
Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R (1995) Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst 87(21):1622–1629
Pettersson A, Hankinson S, Willett W, Lagiou P, Trichopoulos D, Tamimi R (2011) Nondense mammographic area and risk of breast cancer. Breast Cancer Res 13(5):R100
Aitken Z, McCormack VA, Highnam RP, Martin L, Gunasekara A, Melnichouk O, Mawdsley G, Peressotti C, Yaffe M, Boyd NF, dos Santos Silva I (2010) Screen-film mammographic density and breast cancer risk: a comparison of the volumetric standard mammogram form and the interactive threshold measurement methods. Cancer Epidemiol Biomark Prev 19(2):418–428
Stone J, Ding J, Warren RM, Duffy SW, Hopper JL (2010) Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res 12(6):R97
Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, Astrahan M, Siozon CC, Pike MC (2003) Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomark Prev 12(4):332–338
Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN (2005) Mammographic density and breast cancer risk. Am J Epidemiol 162(8):743–752
Boyd N, Martin L, Gunasekara A, Melnichouk O, Maudsley G, Peressotti C, Yaffe M, Minkin S (2009) Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomark Prev 18(6):1754–1762
Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA (2007) Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomark Prev 16(1):43–49
Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS, Czene K, Eriksson L, Hall P, Hartman M, Warren RM, Hislop G, Chiarelli AM, Hopper JL, Krishnan K, Li J, Li Q, Pagano I, Rosner BA, Wong CS, Scott C, Stone J, Maskarinec G, Boyd NF, van Gils CH, Tamimi RM (2014) Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 106(5):dju078
Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, Miglioretti DL (2010) Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol 28(24):3830–3837
Yaghjyan L, Colditz GA, Rosner B, Tamimi RM (2012) Mammographic breast density and breast cancer risk by menopausal status, postmenopausal hormone use and a family history of breast cancer. Cancer Causes Control 23:785
Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM (2011) Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst 103(15):1179–1189
Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz V, Visscher D, Norman A, Couch F, Shepherd J, Fan B, Chen YY, Ma L, Beck AH, Cummings SR, Kerlikowske K, Vachon CM (2013) Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res 15(6):R104
Schnitt SJ (2010) Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol 23(S2):S60–S64
Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan P-H, Tse GM, Badve S, Ellis IO (2010) Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 12(4):1–12
Arpino G, Milano M, De Placido S (2015) Features of aggressive breast cancer. Breast 24(5):594–600
Cianfrocca M, Goldstein LJ (2004) Prognostic and predictive factors in early-stage breast cancer. Oncologist 9(6):606–616
Bentzon N, Düring M, Rasmussen BB, Mouridsen H, Kroman N (2008) Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer 122(5):1089–1094
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295(21):2492–2502
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6
NCCN clinical practice guidelines in oncology: breast cancer. V 1.2016. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 15 Dec 2015
Heine JJ, Scott CG, Sellers TA, Brandt KR, Serie DJ, Wu FF, Morton MJ, Schueler BA, Couch FJ, Olson JE, Pankratz VS, Vachon CM (2012) A novel automated mammographic density measure and breast cancer risk. J Natl Cancer Inst 104(13):1028–1037
Olson JE, Sellers TA, Scott CG, Schueler BA, Brandt KR, Serie DJ, Jensen MR, Wu FF, Morton MJ, Heine JJ, Couch FJ, Pankratz VS, Vachon CM (2012) The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study cohort. Breast Cancer Res 14(6):R147
Tamimi RM, Hankinson SE, Colditz GA, Byrne C (2005) Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomark Prev 14(11 Pt 1):2641–2647
Tworoger SS, Sluss P, Hankinson SE (2006) Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Can Res 66(4):2476–2482
Colditz GA, Hankinson SE (2005) The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 5(5):388–396
Kerlikowske K, Carney PA, Geller B, Mandelson MT, Taplin SH, Malvin K, Ernster V, Urban N, Cutter G, Rosenberg R, Ballard-Barbash R (2000) Performance of screening mammography among women with and without a first-degree relative with breast cancer. Ann Intern Med 133(11):855–863
Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings SR (2005) Are breast density and bone mineral density independent risk factors for breast cancer? J Natl Cancer Inst 97(5):368–374
Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K (2004) Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomark Prev 13(12):2090–2095
Bertrand KA, Rosner B, Eliassen AH, Hankinson SE, Rexrode KM, Willett W, Tamimi RM (2015) Premenopausal plasma 25-hydroxyvitamin D, mammographic density, and risk of breast cancer. Breast Cancer Res Treat 149(2):479–487
Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ (1996) Automated analysis of mammographic densities. Phys Med Biol 41(5):909
Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, Vittinghoff E, Cummings SR (2011) Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomark Prev 20(7):1473–1482
Byng JW, Boyd NF, Little L, Lockwood G, Fishell E, Jong RA, Yaffe MJ (1996) Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev 5(5):319–327
Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE (2000) Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res 60(14):3744–3748
Bagaria SP, Ray PS, Sim MS, Ye X, Shamonki JM, Cui X, Giuliano AE (2014) Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg 149(2):125–129
Arpino G, Bardou VJ, Clark GM, Elledge RM (2004) Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 6(3):R149–R156
Phipps AI, Li CI, Kerlikowske K, Barlow WE, Buist DS (2010) Risk factors for ductal, lobular, and mixed ductal-lobular breast cancer in a screening population. Cancer Epidemiol Biomarkers Prev 19(6):1643–1654
Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, Ellis IO (2005) Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 18(1):26–35
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, O’Meara ES, Li CI (2012) Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol 22(5):340–348
Aiello EJ, Buist DS, White E, Porter PL (2005) Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol Biomark Prev 14(3):662–668
Sala E, Solomon L, Warren R, McCann J, Duffy S, Luben R, Day N (2000) Size, node status and grade of breast tumours: association with mammographic parenchymal patterns. Eur Radiol 10(1):157–161
Ding J, Warren R, Girling A, Thompson D, Easton D (2010) Mammographic density, estrogen receptor status and other breast cancer tumor characteristics. Breast J 16(3):279–289
Ghosh K, Brandt KR, Sellers TA, Reynolds C, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Vachon CM (2008) Association of mammographic density with the pathology of subsequent breast cancer among postmenopausal women. Cancer Epidemiol Biomark Prev 17(4):872–879
Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, White E (2000) Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 92(13):1081–1087
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225(1):165–175
Leconte I, Feger C, Galant C, Berliere M, Berg BV, D’Hoore W, Maldague B (2003) Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol 180(6):1675–1679
Porter GJR, Evans AJ, Cornford EJ, Burrell HC, James JJ, Lee AHS, Chakrabarti J (2007) Influence of mammographic parenchymal pattern in screening-detected and interval invasive breast cancers on pathologic features, mammographic features, and patient survival. Am J Roentgenol 188(3):676–683
Greene F (2002) American Cancer Society, American Joint Committee on Cancer. AJCC cancer staging manual. Springer, New York
Mook S, Schmidt MK, Rutgers EJ, van de Velde AO, Visser O, Rutgers SM, Armstrong N, van’t Veer LJ, Ravdin PM (2009) Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol 10(11):1070–1076
Weigelt B, Baehner FL, Reis-Filho JS (2010) The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol 220(2):263–280
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JEL, Liu ET, Bergh J, Kuznetsov VA, Miller LD (2006) Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Can Res 66(21):10292–10301
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800
Acknowledgements
This study was supported by the National Institutes of Health (Grant Numbers CA140286 to C. M.V. and K.K., CA087969 to R.M.T., UM1 CA176726 to W.W., UM1 CA186107 to M.S.), Mayo Clinic Specialized Program of Research Excellence (SPORE) in Breast Cancer (Grant Number P50 CA116201 to M.P.G. and J.N.I.), Mayo Clinic Cancer Center, and Breast Cancer Research Foundation. We would like to thank the participants and staff of the NHS and NHSII for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Boards at the Mayo Clinic, Brigham and Women’s Hospital, and the University of California, San Francisco. Informed consent was obtained or implied by return of questionnaires (NHS, NHSII).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yaghjyan, L., Tamimi, R.M., Bertrand, K.A. et al. Interaction of mammographic breast density with menopausal status and postmenopausal hormone use in relation to the risk of aggressive breast cancer subtypes. Breast Cancer Res Treat 165, 421–431 (2017). https://doi.org/10.1007/s10549-017-4341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4341-2