Abstract
Purpose
The aim was to evaluate the role of tumor-infiltrating lymphocytes (TIL) in predicting molecular response after preoperative endocrine or cytotoxic treatment for HR+/HER2− patients who do not achieve a pathological complete response.
Methods
Stromal (Str) TIL were centrally evaluated on samples from diagnostic core-biopsies of HR+/HER2− patients included in two prospective randomized trials: the LETLOB trial (neoadjuvant endocrine-based treatment) and the GIOB trial (neoadjuvant chemotherapy-based treatment). Pre- and post-treatment Ki67 was centrally assessed.
Results
StrTIL were evaluable in 111 cases (n = 73 from the LETLOB trial and n = 38 from the GIOB trial). Median StrTIL was 2%. Patients with high StrTIL (StrTIL ≥10%, n = 28) had more frequently breast cancer of ductal histology (p = 0.02), high grade (p = 0.049), and high Ki67 (p = 0.02). After neoadjuvant endocrine treatment (LETLOB cohort), a significant Ki67 suppression (p < 0.01) from pre- to post-treatment was observed in both the low and high StrTIL groups. High StrTIL patients achieve more frequently a relative Ki67 suppression ≥50% from baseline as compared to low StrTIL patients (55 vs. 35%, p non significant). After neoadjuvant chemotherapy (GIOB cohort), a significant Ki67 suppression was observed only for low StrTIL patients (Wilcoxon p = 0.001) and not in the high StrTIL group (p = 0.612). In this cohort, the rate of patients achieving a relative Ki67 suppression ≥50% from baseline was significantly higher in the high vs low StrTIL group (64 vs. 10%, p = 0.003). Geometric mean Ki67 suppression was evaluated in each cohort according to StrTIL: the lowest value (−41%) was observed for high StrTIL cases treated with chemotherapy.
Conclusions
This hypothesis-generating study suggests that in HR+/HER2− breast cancer StrTIL at baseline may influence the achievement of a molecular response after neoadjuvant treatment. Further evaluation in large studies is needed, and interaction with the type of treatment warrants to be explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Neoadjuvant chemotherapy is the standard treatment for locally advanced and inflammatory breast cancer and is being increasingly used for large operable primary tumors in order to allow breast conserving surgery. For postmenopausal hormone-receptor (HR)+/HER2− breast cancer patients, preoperative endocrine treatment with an aromatase inhibitor is a valuable option. Indeed, comparative studies reported similar outcomes in terms of response rate for neoadjuvant chemotherapy vs endocrine treatment. In a recent review, clinical responses for neoadjuvant endocrine treatment vs chemotherapy ranged from 48 to 89% and 64–85%, respectively [1].
Since the achievement of a pathological complete response (pCR) after preoperative treatment has a favorable prognostic value, pCR has been proposed as a surrogate endpoint for long-term survival [2]. However, the rate of pCR after neoadjuvant therapy for HR+/HER2− breast cancer patients is low (around 10%), questioning its surrogacy for this BC subtype [1, 3]. Molecular response as assessed by Ki67% levels on residual disease after preoperative treatment has been reported to correlate with long-term prognosis, most notably in HR+ BC, either after chemotherapy or endocrine therapy [4–6].
HR+/HER2− breast cancer is a heterogeneous disease and such heterogeneity influences response to treatments. Luminal B tumors, as defined by molecular intrinsic subtyping, are known to achieve higher rates of pCR after neoadjuvant chemotherapy compared to Luminal A tumors which, conversely, may be more sensitive to endocrine treatment [7]. However, today there is a lack of finer methods to discriminate luminal-like breast cancer patients who would better benefit from one neoadjuvant approach over another. Moreover, with the recent interest raised around immuno oncology, it is becoming more evident that the interface between tumor and immune cells is an important actor. In their seminal paper, Denkert et al. have shown that tumor-infiltrating lymphocytes (TIL) at baseline predict the achievement of pCR after neoadjuvant chemotherapy in breast cancer patients, including luminal-like [8]. In addition, there is evidence suggesting that not only chemotherapy but also endocrine treatments may interact with the tumor-immune interplay. However, the role of TIL has been underexplored so far for luminal breast cancer [9].
In this paper, we aim to evaluate the role of TIL in predicting molecular response after preoperative endocrine and cytotoxic treatment for HR+/HER2− patients who were included in two closed prospective trials.
Methods
Patients
The LETLOB trial is a double-blind, phase IIb study that randomized 92 postmenopausal HR+/HER2-stage II-IIIA breast cancer patients to receive neoadjuvant letrozole (2.5 mg orally, daily) plus lapatinib (1500 mg orally, daily) or letrozole plus placebo over a 28-days cycle for 6 cycles. Primary endpoint was the objective response rate. Secondary aims included (among others): pCR, breast conserving surgery, safety and inhibition of proliferative biomarkers. Further details and clinical results have been published elsewhere [10]. Briefly, the study results showed that the combination of letrozole-lapatinib is feasible and resulted in similar overall clinical response rate and similar effect on Ki67 compared to letrozole-placebo.
The phase II GIOB trial randomized 90 stage II-IIIA early breast cancer patients to receive chemotherapy with epirubicin 90 mg/m2 intravenous (iv) plus paclitaxel 175 mg/m2 iv on day 1 for 4 courses administered every 3 weeks plus: gefitinib 250 mg orally once daily from day 5 to day 16 of four 3-weekly cycles of chemotherapy (Arm A, intermittent); or gefitinib 250 mg orally once daily from days 1–21 for four 3-weekly cycles of chemotherapy (Arm B, continuous); or placebo orally daily for four 3-weekly cycles of chemotherapy (Arm C, control). The primary end-point was to evaluate the inhibition induced by chemotherapy plus gefitinib vs chemotherapy plus placebo on the EGFR-dependent p42/44 MAPK from biopsy to surgery. Among secondary aims, the evaluation of Ki67 inhibition from biopsy to surgery was planned. Further details and results have been previously published [11]. Briefly, the study showed that adding gefitinib to chemotherapy did not result in different effects on the EGFR-dependent pathway and proliferation. The two schedules of gefitinib (intermittent vs. continuous) did not result in different biologic effects.
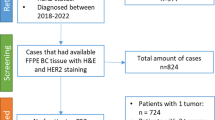
For the purpose of the present analysis, the 92 and the 47 HR+/HER2− patients from the LETLOB and GIOB trials, respectively, were considered (Fig. 1).
The trials were approved by the relevant ethics committees. All patients provided written informed consent before study entry.
Pathology assessments
Formalin-fixed paraffin-embedded tumor samples from both the diagnostic core-biopsy and the surgical sample were centralized at the Division of Pathology of the University Hospital of Modena.
Ki67 was centrally evaluated by immunohistochemistry (IHC). The clone Ki-67-MIB-1 DAKO (Carpinteria, CA) antibody was used. IHC staining was performed according to the avidin–biotin method, using sections of 3 µm of thickness. The % of immunostained tumor cells was recorded.
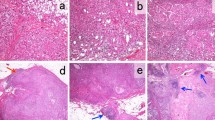
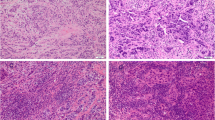
Stromal TIL (StrTIL) were centrally evaluated on hematoxylin and eosin stained (HES) slides from diagnostic core biopsies following consensus guidelines [12].
Estrogen receptor (ER) and progesterone receptor (PgR) were considered positive if IHC staining in ≥10% of tumor cells, according to local assessment. HR positivity was defined as ER and/or PgR ≥10%.
HER2 was considered negative if IHC staining of 0/1+ and/or FISH non-amplified. In the LETLOB trial, central evaluation of HER2 was performed and all primary tumor samples were centrally confirmed as HER2-negative. In the GIOB trial, the HER2 status as assessed by local laboratory was considered since no central review of HER2 status was planned.
Statistical analysis
Statistical analysis was carried out using Statistical analysis using SAS software version 9.3 (SAS Institute Inc., Cary, N.C.) and R Statistical Software [13].
The Pearson’s χ 2 or Fisher Exact tests were used to analyze categorical variables.
Non-parametric test was used to explore the distribution of continuous variables according to baseline clinicopathological characteristics.
Ki67 individual changes were investigated with the Wilcoxon rank signed test. Geometric mean Ki67 suppression was defined as Ln(Ki67post) − Ln(Ki67pre). Student’s t test was used to compare geometric mean Ki67 suppression between groups.
The threshold of 10% was used to categorize patients in StrTILs-defined group. This cut-off is commonly used to separate patients with low StrTILs from patients with intermediate/high StrTILs [14] and corresponded in this patients cohort to the threshold for the upper quartile.
Results
Patients baseline characteristics
Of the 92 and the 47 HR+/HER2− patients from the LETLOB and GIOB trials, n = 111 were evaluable for both StrTIL and Ki67 suppression: 73 patients from the LETLOB trial cohort and 38 patients from the GIOB trial cohort (Fig. 1; evaluable and not evaluable patients were similar according to main clinicopathological features as shown in supplementary data, Online resource 1). Patients’ baseline characteristics are reported in Table 1. As shown, there was no difference between the cohort of patients from the LETLOB and the cohort from the GIOB trial with regard to histotype, tumor size, grade, stage, baseline Ki67, and baseline StrTIL. The two cohorts significantly differed for age, with patients from the LETLOB trial being older than patients from the GIOB trial (postmenopausal status was mandatory for inclusion in the LETLOB study and not for the GIOB study).
Median StrTIL level at baseline was 2% (Q1 0%; Q3 10%). We defined patients in the upper quartile (StrTIL ≥10%, n = 28) as high StrTIL.
Patients’ characteristics according to low and high StrTIL are also reported in Table 1. Almost all cases with high StrTIL were of ductal histology, none of the cases of lobular histology was classified as having high StrTIL. A significant association between high StrTIL levels and higher baseline Ki67 was observed: median Ki67 was 22.5 and 16% in the high StrTIL and low StrTIL groups, respectively (p = 0.02). Moreover, tumors with high StrTIL were more frequently G3 than G1-2 (63 vs. 40%; p = 0.049). These associations are confirmed by the analysis of the distribution of StrTILs as continuous variable according to baseline clinicopathological, as reported in supplementary data, Online resource 2.
Ki67 suppression according to StrTIL
We evaluated the Ki67 individual changes from pre-treatment (diagnostic-core biopsy) to post-treatment (surgery) according to baseline StrTIL levels separately in the two cohort of patients treated with neoadjuvant chemotherapy-based treatment (GIOB) and neoadjuvant endocrine-based therapy (LETLOB). After chemotherapy ± gefitinib a significant Ki67 suppression was observed in case of baseline StrTIL <10% (p = 0.001). In the smaller group of StrTIL ≥10%, a reduction was observed but did not reach statistical significance (p = 0.612; Fig. 2A). As shown in Fig. 2b, after endocrine therapy ± lapatinib a significant Ki67 suppression was observed in both the low and the high StrTIL groups (p < 0.001 and p = 0.001, respectively).
Figure 3 reports individual proportional Ki67 suppression from baseline in the two separate cohorts (GIOB and LETLOB). Proportional Ki67 suppression was calculated as (Ki67post-Ki67pre)/Ki67pre*100. There were no cases of increase in Ki67 among the 18 Str-TIL patients in the LETLOB cohort. The rate of patients showing a proportional Ki67 relative suppression of at least 50% was numerically higher in the high StrTIL group (10/18, 55%) compared to the low StrTIL group (19/55, 35%). In the GIOB cohort, there were 2 out of 10 high-StrTIL cases showing an increase in Ki67. Only 1 of 10 high-StrTIL cases showed a proportional Ki67 reduction by at least 50% from baseline, compared to 18/28 (64%) of low StrTIL cases (p = 0.003). The correlation between StrTIL as continuous variable and proportional Ki67 suppression was also explored and reported as supplementary data (Online resource 3). The two variables showed opposite correlations in the LETLOB (Spearman’s coefficient −0.10) and GIOB (Spearman’s coefficient +0.21) patients cohorts.
The geometric mean Ki67 suppression according to StrTIL in each patients cohort is reported in Fig. 4. The t test comparing the two StrTIL groups was not significant in each trial cohort. However, chemotherapy-treated StrTIL low cases and endocrine-treated StrTIL high cases achieve the highest mean Ki67 suppression (−82 and −81%, respectively). The lowest geometric mean Ki67 suppression (−41%) was observed for StrTIL high cases treated with chemotherapy.
Discussion
This analysis raises the hypothesis that in HR+/HER2− breast cancer the level of StrTIL at baseline may have some influence on the achievement of a molecular response after neoadjuvant treatment, which may differ according to the type of administered therapy (chemotherapy or endocrine treatment). More in detail, high StrTIL tumors were less likely to achieve a molecular response in patients treated with neoadjuvant chemotherapy with residual invasive disease in the breast. To the other hand, high StrTIL levels did not jeopardize molecular response after endocrine treatment: high StrTIL tumors were even numerically more likely to achieve higher Ki67 suppression than low StrTIL.
Luminal-like breast cancer has generally not been considered a highly immunogenic tumor. Indeed, HR+/HER2− tumors are generally less infiltrated by lymphocytes as compared to other subtypes. The low StrTIL levels reported in this work are consistent with previous studies [15, 16]. Although evidence on the prognostic role of TIL has been mainly limited to HR- disease, published data for HR+/HER2− BC suggest no association between TIL and survival in this subgroup [15, 16]. However, it is likely that the role of immunity in HR+/HER2− has been so far underestimated and underexplored. Indeed, despite generally low levels of TIL, heterogeneity has been described, with a few cases presenting massive lymphocyte infiltration, as in our study [17]. ER signaling and endocrine therapies may contribute to modulate the tumor-immune/inflammation milieu with indirect and direct effects on tumor and immune cells [9]. Therefore, the clinical impact of immune related biomarkers in HR+/HER2− BC patients is an area that deserves to be further investigated. In this context, the neoadjuvant setting offers the unique opportunity to test the correlation between biomarkers and response to treatments for primary breast cancer.
With regard to neoadjuvant chemotherapy, previous data indicate that patients with a high level of TIL present higher chance of obtaining a pCR even in case of HR+/HER2− breast [8, 18]. Recently, results from a large series of patients treated with neoadjuvant chemotherapy in the context of prospective trials have been presented. In the HR+/HER2− group (n = 1366), high TIL at baseline not only correlated with a higher chance of pCR, confirming previous results, but were also counterintuitively associated with a worse long-term prognosis in patients with less than pCR [14]. Various explanations may be proposed. Among those, the results of our exploratory study showing that, among HR+/HER2− patients with residual disease after neoadjuvant chemotherapy, those with higher levels of TIL at baseline achieve poorer molecular response may represent one hypothesis to be further tested.
Concerning neoadjuvant endocrine therapy, an analysis conducted on a cohort of 79 HR+ (any HER2) postmenopausal patients treated with anastrozole ± gefitinib reported that cases with detectable lymphocytic infiltration at baseline appeared to have a poorer antiproliferative response at 2 weeks [19], in contrast to our findings. However, differences in patients population (HER2+ patients were included in the analysis by Dunbier et al.), methods of TIL assessment, and timing of Ki67 evaluation may in part explain heterogeneity of the results.
In our study, a significant Ki67 suppression was described irrespectively of StrTIL levels in patients treated with neoadjuvant letrozole. In contrast with other findings, high StrTIL level at baseline did not prevent the achievement of a molecular response. The presence of high levels of TIL may indicate an immune activated tumor but not necessarily an efficient anti-tumor response. In this context, aromatase inhibitors might contribute to switch the tumor-immune microenvironment towards an anti-tumor function through FOXP3+ depletion, as previously described [20]. The final effect on tumor cells might become evident later than few weeks from the start of treatment.
This study has limitations. First, the sample size is small and results can only be considered as hypothesis-generating. Furthermore, the two trial populations differ according to age and menopausal status; therefore, results from single cohorts should not be generalized or directly compared. It is still unknown whether and to which extent menopausal status influences the tumor-immune microenvironment. Another limitation is that some of the patients also received lapatinib or gefitinib in addition to endocrine therapy or chemotherapy, respectively. However, no interference with the evaluation of Ki67 suppression was expected, as neither lapatinib nor gefitinib modified Ki67 suppression in the original studies [10, 11].
Despite these limitations, this study is based on prospectively collected data from two randomized clinical trials, in which Ki67 evaluation was preplanned and centralized. StrTIL evaluation was also centralized for both studies.
In conclusion, this paper represents the first evidence evaluating, for HR+/HER2- breast cancer, the effect of neoadjuvant chemotherapy and endocrine therapy on molecular response after treatment completion in relation with baseline TIL levels. The results highlight the need to further explore the correlation between immune aspects and HR+/HER2− breast cancer, and suggest that treatment-related factors should not be overlooked in such studies.
References
Charehbili A, Fontein DB, Kroep JR, Liefers GJ, Mieog JS, Nortier JW, van de Velde CJ (2014) Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: a systematic review. Cancer Treat Rev 40:86–92. doi:10.1016/j.ctrv.2013.06.00
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. doi:10.1016/S0140-6736(13)62422-8
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. doi:10.1200/JCO.2011.38.8595
Guarneri V, Piacentini F, Ficarra G, Frassoldati A, D’Amico R, Giovannelli S, Maiorana A, Jovic G, Conte P (2009) A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol 20:1193–1198. doi:10.1093/annonc/mdn761
von Minckwitz G, Schmitt WD, Loibl S, Müller BM, Blohmer JU, Sinn BV, Eidtmann H, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, Fehm T, Barinoff J, Rüdiger T, Erbstoesser E, Fasching PA, Karn T, Müller V, Jackisch C, Denkert C (2013) Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res 19:4521–4531. doi:10.1158/1078-0432.CCR-12-3628
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W, Dowsett M (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100:1380–1388. doi:10.1093/jnci/djn309
Prat A, Fan C, Fernandez A, Hoadley KA, Martinello R, Vidal M, Viladot M, Pineda E, Arance A, Muñoz M, Paré L, Cheang MC, Adamo B, Perou CM (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13:303. doi:10.1186/s12916-015-0540-z
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113. doi:10.1200/JCO.2009.23.7370
Dieci MV, Griguolo G, Miglietta F, Guarneri V (2016) The immune system and hormone-receptor positive breast cancer: is it really a dead end? Cancer Treat Rev 46:9–19. doi:10.1016/j.ctrv.2016.03.011
Guarneri V, Generali DG, Frassoldati A, Artioli F, Boni C, Cavanna L, Tagliafico E, Maiorana A, Bottini A, Cagossi K, Bisagni G, Piacentini F, Ficarra G, Bettelli S, Roncaglia E, Nuzzo S, Swaby R, Ellis C, Holford C, Conte P (2014) Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J Clin Oncol 32:1050–1057. doi:10.1200/JCO.2013.51.4737
Guarneri V, Frassoldati A, Ficarra G, Puglisi F, Andreetta C, Michelotti A, Cresti N, Boni C, Bisagni G, Berardi R, Battelli N, Santoro A, Banna G, Bottini A, Di Blasio B, Maiorana A, Piacentini F, Giovannelli S, Jovic G, Conte P (2008) Phase II, randomized trial of preoperative epirubicin-paclitaxel ± gefitinib with biomarker evaluation in operable breast cancer. Breast Cancer Res Treat 110:127–134. doi:10.1007/s10549-007-9688-3
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. doi:10.1093/annonc/mdu450
R Development Core Team (2016) R R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.rproject.org/. Accessed on June 2016
Denkert C, von Minckwitz G, Darb-Esfahani S, Ingold Heppner B, Klauschen F, Furlanetto J, Pfitzner B, Huober J, Schmitt W, Blohmer J-U, Kümmel S, Engels K, Lederer B, Schneeweiss A, Hartmann A, Jakisch C, Untch M, Hanusch C, Weber K, Loibl S (2016) Evaluation of tumor-infiltrating lymphocytes (TILs) as predictive and prognostic biomarker in different subtypes of breast cancer treated with neoadjuvant therapy—a metaanalysis of 3771 patients. Abstract S1-09. Presented at the 39th San Antonio breast cancer symposium, December 6–10 2016, San Antonio, Texas, USA
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867. doi:10.1200/JCO.2011.41.0902
Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, Goubar A (2015) Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 26:1698–1704. doi:10.1093/annonc/mdv239
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2:1354–1360. doi:10.1001/jamaoncol.2016.1061
Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, Sinn BV, Ulmer HU, Kronenwett R, Just M, Kühn T, Diebold K, Untch M, Holms F, Blohmer JU, Habeck JO, Dietel M, Overkamp F, Krabisch P, von Minckwitz G, Denkert C (2013) Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE 8:e79775. doi:10.1371/journal.pone.0079775
Dunbier AK, Ghazoui Z, Anderson H, Salter J, Nerurkar A, Osin P, A’hern R, Miller WR, Smith IE, Dowsett M (2013) Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin Cancer Res 19:2775–2786. doi:10.1158/1078-0432.CCR-12-1000
Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Banham AH, Harris AL, Bottini A, Fox SB (2009) Immunomodulation of FOXP3 + regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res 15:1046–1051. doi:10.1158/1078-0432.CCR-08-1507
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The LETLOB study was sponsored and funded by GlaxoSmithKline; the GIOB study was sponsored and funded by Astrazeneca. Moreover, authors acknowledge the following fundings: Istituto Oncologico Veneto project L02P38; University of Padova project 60A07-7808/13.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10549-017-4219-3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dieci, M.V., Frassoldati, A., Generali, D. et al. Tumor-infiltrating lymphocytes and molecular response after neoadjuvant therapy for HR+/HER2− breast cancer: results from two prospective trials. Breast Cancer Res Treat 163, 295–302 (2017). https://doi.org/10.1007/s10549-017-4191-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4191-y