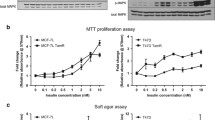
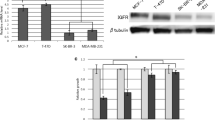
Abstract
Purpose
Interactions between HER2, estrogen receptor (ER), and insulin-like growth factor I receptor (IGF1R) are implicated in resistance to monotherapies targeting these receptors. We have previously shown in pre-clinical studies synergistic anti-tumor effects for co-targeting each pairwise combination of HER2, IGF1R, and ER. Strikingly, synergy for HER2/IGF1R targeting occurred not only in a HER2+ model, but also in a HER2-normal model. The purpose of the current study was therefore to determine the generalizability of synergistic anti-tumor effects of co-targeting HER2/IGF1R, the anti-tumor activity of triple-targeting HER2/IGF1R/ER in hormone-dependent cell lines, and the effect of using the multi-targeting drugs neratinib (pan-HER) and BMS-754807 (dual IGF1R/insulin receptor).
Methods
Proliferation and apoptosis assays were performed in a large panel of cell lines representing varying receptor expression levels. Mechanistic effects were studied using phospho-protein immunoblotting. Analyses of drug interaction effects were performed using linear mixed-effects regression models.
Results
Enhanced anti-proliferative effects of HER/IGF-insulin co-targeting were seen in most, though not all, cell lines, including HER2-normal lines. For ER+ lines, triple targeting with inclusion of anti-estrogen generally resulted in the greatest anti-tumor effects. Double or triple targeting generally resulted in marked increases in apoptosis in the sensitive lines. Mechanistic studies demonstrated that the synergy between drugs was correlated with maximal inhibition of Akt and ERK pathway signaling.
Conclusions
Dual HER/IGF-insulin targeting, and triple targeting with inclusion of anti-estrogen drugs, shows striking anti-tumor activity across breast cancer types, and drugs with broader receptor specificity may be more effective than single receptor selective drugs, particularly for ER− cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
There is cross-talk between HER2 and ER signaling in breast cancer, and signaling by HER2/HER family receptors is a mechanism of endocrine resistance [1]. We have previously shown that in the ER+, HER2-overexpressing human breast cancer BT474 cell line, the combination of trastuzumab plus tamoxifen results in synergistic anti-proliferative effects, enhanced effects on cell cycle, and reduction of clonogenicity, but not induction of apoptosis [2]. However, combining a dual HER2/EGFR inhibitor with anti-estrogens did induce apoptosis, and adding HER2 or dual HER2/EGFR targeting drugs to anti-estrogens in the MCF7 ER+ HER2-normal model elicited enhanced anti-tumor effects for HER2/ER co-targeting that were not dependent upon HER2 overexpression, including both inhibition of proliferation and induction of apoptosis [3].
Some potential mechanisms of intrinsic or acquired resistance to trastuzumab have been discovered. These include tumor expression of truncated or cleaved forms of constitutively activated HER2 lacking the trastuzumab-binding extracellular domain [4, 5], constitutive activation of the PI3K pathway [6–11], expression of MUC4 [12, 13], signaling via src [14–16], a HER2 splice variant lacking a 16 amino acid exon in the extracellular domain [14, 17], enhanced activity of other HER/EGFR family receptors and/or expression of their ligands [18–21], and signaling by the insulin-like growth factor I receptor (IGF1R) [22–25]. In addition, enhanced ER signaling has been implicated as a potential mechanism of resistance to HER2-directed therapy, particularly for the HER2/EGFR dual tyrosine kinase inhibitor (TKI) lapatinib [26, 27]. Hence, either HER2 or ER can mediate resistance to agents targeting the other receptor system.
Several clinical trials have studied the effect of co-targeting HER2 and ER in breast cancer patients with HER2+ or HER2-normal tumors, with somewhat disappointing results [28–30]. Unlike what was expected from pre-clinical models, thus far clinical studies have not shown benefit for targeting HER2 in combination with endocrine therapy for HER2-normal breast cancer, suggesting that additional escape mechanisms exist. One such escape mechanism may be IGF1R signaling. The IGF1R interacts with both HER2 and ER signaling, and IGF1R has been implicated in resistance to both HER2-directed therapy [22, 24, 31, 32] and ER-directed therapy [33–35], and in doubly anti-estrogen/anti-HER family drug resistance [36].
Cross-talk and physical interaction of IGF1R with HER2 has been described [31, 37]. Experimental overexpression of IGF1R results in IGF-I-mediated trastuzumab resistance [24, 38]; cells cultured long-term in trastuzumab to induce acquired resistance up-regulated endogenous IGF1R, acquired a physical association between HER2 and IGF1R, and IGF was able to induce HER2 cross-phosphorylation [31]. Clinically IGF1R has been found to be associated with resistance to trastuzumab [22]. Bi-directional cross-talk and physical interaction of IGF1R with ER has also been described [33–35, 39–49]. A kinome-wide siRNA screen identified IGF1R and insulin signaling as means of escape from the effect of estrogen deprivation in estrogen-dependent breast cancer cells [32].
Co-targeting IGF1R is therefore a logical strategy to enhance therapy aimed at targeting HER2, ER, or their combination. IGF1R-targeted drugs to date have generally not shown significant single agent activity. However, the anti-apoptotic role of IGF1R in established tumors suggests that it may be more effective to combine IGF1R targeting with other therapies. We previously described co-targeting HER2 and IGF1R in two cell culture models: BT474 (ER+, HER2+) and MCF7 (ER+, HER2-normal). We found that such dual targeting resulted in disruption of HER2/IGF1R cross-talk, synergistic inhibition of cell proliferation, and induction of cellular apoptosis in the HER2-overexpressing BT474 cell line model; most remarkably, we also found that the cytotoxic effects of the HER2/IGF1R combination targeting occurred as well in the HER2-normal MCF7 cell line; HER2 over-expression was not required for the synergistic killing effects of HER2/IGF1R co-targeting [3, 50]. Targeting IGF1R was able to bring about a dramatic anti-tumor effect of trastuzumab in a HER2-normal tumor model. These effects were also reproduced using in vivo tumor xenografts with these two cell line models [3].
We have similarly described anti-tumor effects of co-targeting ER and IGF1R. We found that in vitro, co-targeting ER/HER2 resulted in synergistic inhibition of cell proliferation, but was cytostatic only, without induction of apoptosis [2, 51]. We found that co-targeting ER/IGF1R also produced enhanced inhibition of cell proliferation, but one could now observe induction of apoptosis; in addition, ER/IGF1R crosstalk was disrupted by co-targeting both receptor systems [52].
Early clinical trial results have been reported for co-targeting IGF1R with ER or HER2, with disappointing results. The addition of the IGF1R therapeutic monoclonal antibody AMG 479 to exemestane or fulvestrant did not improve outcomes [53]. Similarly, the addition of the IGF1R antibody ganitumab to exemestane or fulvestrant did not improve outcome [54]. The IGF1R therapeutic monoclonal antibody cixutumumab did not improve the outcome of treatment with lapatinib/capecitabine for HER2+ breast cancer patients previously treated with trastuzumab [55].
With respect to targeting ER, HER2, and IGF1R, it is not clear why in the clinic disappointingly various two-drug combinations are not giving more robust clinical results. We address two potential reasons in the current work. We hypothesize that the first reason is the 3-way network of cross-talk amongst ER, HER2, and IGF1R, providing escape mechanisms leading to resistance when any two of these receptor systems are targeted. Hence, here we describe experiments of triple-targeting ER, HER2, and IGF1R.
A second potential reason for lack of more robust clinical results with HER2 or IGF1R targeting drugs involves the contribution partner receptors. IGF1R may co-signal with the insulin receptor (IR) and IGF1R and IR are highly homologous, have some overlapping functions, and are capable of forming hybrid receptors that can be activated by ligands for either, signaling through both. Compelling experimental evidence supports the hypothesis that both of these receptors may need to be targeted to realize the full anti-tumor effect of IGF1R-directed therapy [32, 56]. We therefore compared the efficacy of a dual IGF1R/IR-targeting drug, BMS-754807, to selective IGF1R targeting. Similarly, since trastuzumab resistance may also arise from excess signaling by other HER family receptors, one approach to overcoming trastuzumab resistance is the simultaneous inhibition of multiple HER family receptors. Therefore, we also explored the anti-tumor activity of a pan-HER family receptor TKI, neratinib (HKI-272).
Our previous results in a limited number of breast cancer cell line models described above showed that synergy in co-targeting HER2 and IGF1R was not dependent upon HER2 overexpression, but occurred even in HER2-normal cell lines, both in vitro and in vivo. The goals of the present studies were therefore to (1) determine how generalizable this phenomenon may be by examining a larger panel of breast cancer cell lines representing various patterns of receptor level of expression; (2) to study the effects of pan-HER (neratinib) and IGF1R/IR dual targeting (BMS-754807) drugs in this regard, and (3) to examine the effect of triple-targeting HER2/ER/IGF1R in breast cancer models.
Methods
Cell lines
MCF7 cells were a gift from Dr. Marc Lippman (University of Michigan). All other cell lines were obtained from ATCC. For cell lines from ATCC, cells were carried not more than 10 passages and not more than 3 months before using new frozen stocks; therefore, repeat authentication in our local laboratory was not performed; ATCC authenticates cell lines using STR analysis (DNA profiling).
Cell culture
MCF7 cells were maintained in phenol red-free IMEM with 10% charcoal-stripped fetal calf serum (Biosource), 1 nM estradiol added back, and penicillin, at 37 °C in 5% CO2 humidified air; to maintain sensitivity of the MCF7 cells to the effects of estrogen and anti-estrogens, prior to each experiment cells were cultured for 5 days in estrogen-free medium; estradiol was then added back, with or without experimental drugs, at the beginning of each experiment. BT474, BT549, MDA-MB-231, MDA-MB-453, T47D, ZR-75-1, and ZR-75-30 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (heat inactivated), 2 mM l-glutamine, 10 µg/ml insulin, and penicillin at 37 °C in 5% CO2 humidified air. BT20, MDA-MB-468, and MDA-MB-361 were cultured in EMEM + Ins + Glu, DMEM/F12 + Ins + Glu, and MEM + Ins + Glu, respectively.
Drugs
4-hydroxy tamoxifen (4HT) was purchased from Sigma (St. Louis, MO). Fulvestrant (Faslodex, ICI 182,780) was obtained from the Yale Cancer Center Medical Oncology pharmacy. Trastuzumab was obtained from the Yale Cancer Center Medical Oncology pharmacy. BMS-754807 was a gift from Bristol Myers Squibb (Plainsboro, NJ). Figitumumab (CP-751,871) and neratinib were gifts from Pfizer Inc. (Groton, CT).
Antibodies
Rabbit polyclonal antibody against AKT, phospho-AKT, ERK1/2, phospho-ERK1/2, and PARP (poly ADP-ribose polymerase) were from Cell Signaling Technology (Beverly, MA). HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Anti-ß-actin mouse monoclonal antibody was from Sigma.
Assays of proliferation and apoptosis
Cell proliferation was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). For in vitro growth assays, cells (1 × 103/well) were plated in 96-well plates and treated with the indicated concentrations of the inhibitors for 5 days (time point chosen to be prior to plateau for controls). On Day 6, luminescence was read using the Envision plate reader. Results were expressed as percentage of control (vehicle DMSO-treated) cells. Results presented are mean ± SD from three separate experiments done in triplicate. Apoptosis was assayed by the Annexin V assay, and by the PARP cleavage assay, as described previously [50].
Immunoblotting
Cell extracts (40 µg protein/lane) were immunoblotted with primary antibodies at a dilution of 1:1000, followed by HRP-conjugated respective secondary antibody (1:1000 dilution). Bands were visualized with the enhanced chemiluminescence reagent (Amersham Pharmacia Biotech) on X-ray film (Eastman Kodak, Rochester, NY). ß-actin was used for loading control.
Analysis of drug interaction effects
Linear mixed-effects (LME) regression models were used to evaluate the significance of superadditive synergistic effects of doublet and triplet drug combinations for each cell line. All models had random intercepts to account for variability between the triplicate experiments. The fixed effects included terms to model the main effect of individual drugs and interaction terms to model the effect of drug combinations. The three within-experiment replicates were used to estimate within experiment error. All computations were performed in R v. 3.0.2 [57]. LME regression analysis was performed using package lme4 [58] and the 95% confidence intervals of the estimates were computed from the likelihood profile.
Results
Cell lines and drugs
A panel of representative breast cancer cell lines (Table 1) was chosen to represent varying receptor levels for HER2, IGF1R, and ER, as reported [59, 60]. Trastuzumab is a humanized monoclonal antibody against HER2, while neratinib is a pan-HER family TKI [61, 62]. Figitumumab (CP-751,871) is a humanized monoclonal antibody against IGF1R [63], while BMS-754807 is a dual-specificity TKI that inhibits both IGF1R and insulin receptor. 4HT is a selective ER modulator (SERM), while fulvestrant is a selective ER down-regulator (SERD) with pure antagonist effect against ER.
Effect of drugs on cell proliferation
The effects of single, double, and triple drug targeting of the HER, IGF/insulin, and ER receptors were analyzed in the panel of breast cancer cell lines; for ER-lines, only targeting of HER and IGF/insulin was performed. Preliminary dose–response experiments were performed for each drug in each cell line (not shown). For the testing of drug interactions using drug combinations, drugs were used at approximately the IC75 dose for each individual cell line, and therefore, drug concentrations in the combination studies may differ between cell lines; the actual drug concentrations used are shown in Fig. 1. In some circumstances, a given cell line was resistant to a drug; in that case, for combination studies, the drug to which the line was resistant was used at a concentration at the upper range of the dose–response curve for cell lines sensitive to that drug.
Anti-proliferative effects of drugs against panel of cell lines. The upper panel shows the anti-proliferative effects of drugs against ER− cell lines, and the lower panel against ER+ cell lines. H trastuzumab (Herceptin), N neratinib, CP figitumumab (CP-751,871), BMS BMS-754807, 4HT 4-hydroxy tamoxifen, F fulvestrant, V vehicle treated. Concentrations used for each cell line are indicated in each panel. Percent of control (vehicle treated) proliferation is given at the top of each bar. Error bars are SDs from three different assays each done in triplicate
For ER− cell lines, in general, double targeting of HER and IGF/insulin receptor families resulted in greater anti-proliferative effects than single drug therapy (Figs. 1, 2). However, this was not the case for all cell lines; BT20 cells showed little anti-proliferative response for single or double drug treatments. Some cell lines showed dramatic responses for double drug therapy compared to single drug therapy (e.g., MDA-MB-231 and MDA-MB-453 cell lines). In general, as single drug therapy and in combinations, the pan-HER TKI neratinib showed greater anti-proliferative effects compared with trastuzumab, and the dual IGF1R/insulin receptor TKI BMS-754807 showed greater anti-proliferative effects compared with the IGF1R antibody figitumumab; the combination of neratinib plus BMS-754807 typically produced the greatest anti-proliferative effect. The effects of drug combinations were not predictable from receptor expression levels or from effects of single drugs; for example, the MDA-MB-231 cell line has low level expression of HER2, and would not be expected to respond to HER2-directed drugs, but shows striking effects of combining HER2-directed drugs with IGF1R/insulin-directed drugs.
Two-way interaction plots for visualizing anti-proliferative effects of doublet drug combinations. Shown are data for ER− (panel A) and ER+ (panel B) cell lines. The top row shows single-agent effects and points on the grid show the effect of the corresponding doublet combination. The average anti-proliferative effect is proportional to the diameter of the filled circle and the open (red) circle shows its 95% confidence upper limit
For ER+ cell lines, in general, double targeting resulted in greater anti-proliferative effects than single drug therapy, whether the dual targeting was HER and IGF/insulin receptor pairing, ER and HER pairing, or ER and IGF/insulin pairing (Figs. 1, 2), and in general triple targeting gave greater anti-proliferative effects than double targeting (Figs. 1, 3). Once again, the degree of combinatorial effect differed for different cell lines, with, for example, ZR-75-30 and MCF7 cell lines being particularly sensitive but ZR-75-1 cells considerably less sensitive to the effect of drug combinations.
Three-way interaction plots for visualizing anti-proliferative effects of triplet drug combinations. Shown are data for ER+ cell lines. Each triangular region on this plot corresponds to a triplet drug combination. The effect of the doublet combinations is shown on the edge connecting the two drugs and that of the triplet combination is shown in the middle of the triangle having the three drugs at its vertices. The average anti-proliferative effect is proportional to the diameter of the filled circle and the open (red) circle shows its 95% confidence upper limit
For most of the ER+ lines, there was little difference in doublet efficacy between trastuzumab and neratinib; however, neratinib gave greater anti-proliferative effects compared with trastuzumab in MCF7 cells, while the reverse was the case in ZR-75-30 cells (Figs. 1, 2). The same was true for the triple-targeting therapies, though neratinib also had somewhat greater effect than trastuzumab for the ZR-75-1 cell line (Figs. 1, 3).
For ER+ lines, there was generally a slightly greater effect with doublet therapy for BMS-754807 compared to figitumumab, except for MDA-MB-361 cells for which it was similar (Figs. 1, 2). For triple-targeting therapies, there was a slightly greater effect for BMS-754807 as well (Figs. 1, 3).
For some of the ER+ cell lines (T47D, MCF7, ZR-75-30), fulvestrant had slightly greater anti-proliferative effect compared with 4HT in doublet therapy (Figs. 1, 2). For most of the ER+ cell lines, fulvestrant had the greater effect in triple combination treatments (Figs. 1, 3).
Linear mixed effects (LME) regression analysis was used to assess the significance of single agent effects and of the degree of drug interaction and synergism for drug combinations for anti-proliferative effects, accounting for variation observed within and between experiments (Fig. 4). The “main effect” of individual drugs shown at the top of each forest plot represents the average percent inhibition of the drug relative to vehicle control. Interaction terms capture the effect of the first drug on the main effect of the second drug, and vice versa. Statistically, an interaction term is defined as the difference between the main effect of the second drug when given together with the first drug minus the main effect of the second drug alone. A zero interaction term between two drugs implies that the main effect of either drug is not influenced by the other drug or that the anti-proliferative activity of the two drugs is additive. Thus, a positive interaction term implies a synergistic combination and negative interaction an antagonistic. Three-way interactions are interpreted in a similar way: they capture the effect of a drug on the two-way interaction of the other two drugs. If the estimated 95% confidence interval of a term does not cross 0, the effect is considered statistically significant. For example as seen in Fig. 4B, trastuzumab has a significant effect on ZR-75-30 cells (32.7) but fulvestrant has a small, insignificant effect (2.3). Yet, the interaction of these two drugs (39.1) is highly significant, suggesting strong synergy between fulvestrant and trastuzumab in ZR-75-30. However, addition of BMS-754807 reduces the interaction between fulvestrant and trastuzumab to 9.7, resulting in a negative 3-way interaction (−29.3). Therefore, although BMS-754807 contributes to the anti-proliferative effect of triplet therapy with trastuzumab and fulvestrant, its effect is sub-additive. This may be due to saturation as the triplet therapy resulted in 100% inhibition of the cell line.
Forest plots of the parameters estimated from the linear mixed-effects regression analysis. Shown are data for the ER− (panel A) and the ER+ (panel B) cell lines. The size of the box is proportional to the precision of the estimate and the lines show the 95% confidence interval of the estimate computed by the likelihood profile of the mixed model. An effect is significant at the 5% level if its confidence interval does not include 0
ER− cell lines generally showed sensitivity to all single agents, except for BT20 cells which were not sensitive to any single agent or doublet therapies (Fig. 4A). (Recall however the caveat that different drug concentrations were used for each cell line.) Using the LME regression analysis to analyze drug interactions, among the HER targeting drugs, trastuzumab generally did not show synergy in doublet therapy when paired with either of the IGFR inhibitors, but neratinib showed significant synergy with both (Fig. 4A). Interestingly, although figitumumab was not effective as a single agent in BT549 cells, it had a significant superadditive synergistic effect in combination with neratinib (Fig. 4A).
ER+ cell lines were generally sensitive to single agent treatments, with MDA-MB-361 cells being the most sensitive (Fig. 4B), although doublet or triplet therapies did not show any significant synergistic effects in MDA-MB-361 cells. (Recall again the caveat that different drug concentrations were used for each cell line.) BT474 and MCF7 cells appeared to show the greatest drug combinatorial effects for doublet treatments. Neratinib appeared more synergistic when paired with BMS-754807 compared to figitumumab, but not with either of the ER-directed treatments. Trastuzumab was also slightly more synergistic with BMS-754807 compared to figitumumab and also when paired with 4HT compared to fulvestrant. Triple combination treatments were generally effective but only combinations involving neratinib and fulvestrant with either BMS-754807 or figitumumab showed significantly superadditive synergistic effects in some of the ER+ cell lines (Fig. 4B). Note that in such analyses, it is statistically a large hurdle to demonstrate synergy of adding a third drug to a two-drug combination.
Induction of apoptosis
For selected cell lines displaying the most significant effects of drug combinations, apoptosis assays were conducted to compare the ability of singlet versus combinations. For the ER− cell line MDA-MB-231, single agent treatment with trastuzumab, neratinib, or BMS-754807 did not elicit levels of apoptosis above the background, but adding BMS-754807 to either trastuzumab or neratinib resulted in marked levels of apoptosis, with approximately 90% of cells undergoing apoptosis by Annexin V assay (Fig. 5). Greater levels of apoptosis with the combination treatment were also observed by PARP cleavage assay (Fig. 5). For ER− MDA-MB-453 cells, the same pattern held true although the absolute levels of apoptosis were somewhat less. Greater levels of apoptosis with the combination treatment were also observed by PARP cleavage (Fig. 5). In contrast, the same drug combinations did not significantly induce apoptosis in the BT20 cell line by either assay (Fig. 5).
Effect of drugs on induction of apoptosis in selected cell lines. Apoptosis was measured for indicated cell lines by the Annexin V (panels A and B) and PARP cleavage (panels C and D) assays. V vehicle, H trastuzumab (Herceptin), N neratinib, F fulvestrant, BMS or B BMS-754807. Each data point represents the mean ± SD of 3 different assays done in duplicate, analyzed using the two-tailed paired t test (Abacus, STATVIEW Program). In bar graphs, the asterisks *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.005, respectively
For ER+ BT474 cells, single agent BMS-754807 or fulvestrant did not elicit levels of apoptosis above the background, while trastuzumab and neratinib as single agents caused a small degree of increased apoptosis (Fig. 5); adding BMS-754807 to the HER2-targeting drugs or to fulvestrant resulted in marked levels of apoptosis by Annexin V assay, which were even higher for the triple drug combinations. When analyzed by PARP cleavage, generally enhanced apoptosis was seen for doublet therapy, and the greatest degrees of apoptosis observed when BMS-754807 was used in triplet therapy (Fig. 5). For ER+ ZR-75-30 cells, single agent BMS-754807 did cause a moderate increase in apoptosis above background (Fig. 5), which was slightly higher when combined with trastuzumab, neratinib, or fulvestrant, and marginally higher for the BMS-754807/neratinib/fulvestrant triplet. For MCF7 cells (Fig. 5), similar patterns were observed, with moderate increases above background for doublet therapies, and the highest levels for triple drug therapy. When analyzed by PARP cleavage (Fig. 5), generally enhanced apoptosis was seen for doublet therapy, and the greatest degrees of apoptosis observed when BMS-754807 was used in triplet therapy.
Effects on signaling
For selected cell lines of interest displaying the greatest effects of drug combinations, receptor downstream signaling effects of singlet versus combinations were analyzed by immunoblotting for levels of phosphorylated ERK and Akt. For the ER− cell lines MDA-MB-231 and MDA-MB-435, single agent HER or IGF/insulin targeting drugs caused little to no suppression of levels of phosphorylated ERK or Akt (Fig. 6). However, the addition of BMS-754807 to either trastuzumab or neratinib elicited the greatest suppression of levels of phospho-ERK and- Akt in both cell lines. Such effects were not observed for the BT20 cell line (Fig. 6). Hence, the anti-tumor effects in these cell lines correlate with the ability of the drug combinations to suppress ERK and Akt signaling activity.
For ER+ BT474 cells, adding BMS-754807 to trastuzumab or neratinib caused significant decreases in phospho-ERK and phospho-Akt, and triple targeting with fulvestrant caused the most marked decreases (Fig. 6). In MCF7 and ZR-75-30 cells, the triple therapy also caused the greatest decreases in phospho-ERK and Akt (Fig. 6).
Discussion
HER2, ER, and IGF1R signaling pathways interact extensively, and resistance to therapeutic targeting of any one of these signaling pathways in breast cancer can be achieved by escape signaling in another. We previously published studies of the anti-tumor effects of co-targeting each possible dual-wise pairing of ER, HER2, and IGF1R. We found that in vitro, co-targeting ER/HER2 resulted in synergistic inhibition of cell proliferation, but was cytostatic only, without induction of apoptosis [2, 51]. Co-targeting ER/IGF1R produced enhanced inhibition of cell proliferation, and one could now observe induction of apoptosis; ER/IGF1R crosstalk was disrupted by co-targeting [52]. Likewise, co-targeting HER2 and IGF1R resulted in disruption of crosstalk, synergistic inhibition of cell proliferation, and also induction of significant levels of apoptosis [50]; most remarkably, these results occurred even in breast cancer cells that do not have amplification or overexpression of HER2 (MCF7 cells): HER2 over-expression was not required for the synergistic killing effects of HER2/IGF1R co-targeting [50]. We have published proof of concept in vivo experiments to further support these initial observations.
Our results suggested that (1) co-targeting IGF1R in addition to HER2 is a promising strategy for treating HER2+ breast tumors; (2) this strategy may also extend to HER2 “negative” (i.e., HER2-normal) breast cancer; and (3) triple-targeting IGF1R, HER2, and ER may be the best anti-tumor strategy for ER+ breast cancers. The purpose of the present studies was to determine how generalizable these phenomena may be by examining a larger panel of breast cancer cell lines representing different breast cancer subtypes, and to examine the effect of triple-targeting HER2/ER/IGF1R. In addition, we sought to study the effects of pan-HER (neratinib) and IGF1R/IR dual targeting (BMS-754807) drugs since signaling by other HER family receptors may mediate trastuzumab resistance, and inhibiting the IR may be necessary for a robust anti-tumor effect of IGF1R targeting.
Our current results confirm the generalizability of our prior observations, but not their universality. For 4 of the 5 ER− cell lines examined, we find enhanced effects of co-targeting HER2 and IGF1R, once again even in cell lines with low HER2 expression; however, one cell line (BT20) was resistant to single agents or any doublet combination. For the sensitive ER− cell lines, generally greater effects were seen for the multiple receptor-targeting drugs (neratinib, BMS-754807) compared to the mono-receptor-specific drugs (trastuzumab, figitumumab). In ER+ cell lines, generally double and triple targeting produced incrementally superior results, although the degree to which drug combinations enhanced effects was variable amongst the cell lines, and the potential advantage of the multiple receptor-targeting drugs (neratinib, BMS-754807) was not as consistent as it was for ER− cell lines.
The full factorial designs that we employed in this study allowed us to apply standard statistical interaction models to evaluate the significance of superadditive or synergistic effects of combinations of treatments compared to the effects of individual agents when used alone. Our results suggest that selected targeted therapy drug combinations may have dramatic synergy, even in settings where single drugs are inactive, or where their activity is unexpected (e.g., targeting HER2 in HER2-normal tumors). One hurdle proposed for lack of more success in clinical trials of IGF-targeting drugs is lack of biomarkers to guide patient selection. However, success may also come from optimizing drug combinations, and disabling the insulin-IGF axis. Browne et al. showed enhanced effects of combining trastuzumab with IGF1R inhibiting drugs in a panel of HER2+ cell lines [64], and the levels of IGF1R or phosphorylated IGF1R were not predictive of the response to the combinations. Hou et al. have reported that BMS-754807 synergizes in vitro and in vivo with tamoxifen, letrozole, or fulvestrant in breast cancer models [65]; in addition, the combination resulted in upregulation of HER2, EGFR, and HER4, supporting our proposal that triple-targeting, and inhibiting the entire HER axis, would be of benefit. Our results support the notion that co-targeting of the IGF/insulin-signaling axis and HER-family axis may hold promise in treating breast cancers of various subtypes, and triple targeting may be of benefit for a broad spectrum of ER+ breast tumors.
Abbreviations
- ER:
-
Estrogen receptor
- IGF1R:
-
Insulin-like growth factor I receptor
- IGF:
-
Insulin-like growth factor
- PI3K:
-
Phosphoinositide 3-kinase
- TKI:
-
Tyrosine kinase inhibitor
- 4HT:
-
4-Hydroxy tamoxifen
- LME:
-
Linear mixed-effects
- SERM:
-
Selective estrogen receptor modulator
- SERD:
-
Selective estrogen receptor down-regulator
- HER2:
-
Human epidermal growth factor-like receptor 2
References
Ma CX, Reinert T, Chmielewska I, Ellis MJ (2015) Mechanisms of aromatase inhibitor reistance. Nat Rev Cancer 15:261–275
Argiris A, Wang C-X, Whalen SG, DiGiovanna MP (2004) Synergistic interactions between tamoxifen and trastuzumab (Herceptin). Clin Cancer Res 10:1409–1420
Chakraborty AK, Mehra R, DiGiovanna MP (2015) Co-targeting ER and HER family receptors induces apoptosis in HER2-normal or overexpressing breast cancer models. Anticancer Res 35:1243–1250
Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, DiCosimo S, Matias-Guiu X, Ramon Y, Cajal S, Arriba J et al (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99(8):628–638
Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, Clinton GM (1998) NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 58:5123–5129
Berns K, Horlings HM, Hennessey BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzelez-Angulo AM, Stemke-Hale K, Hauptmann M et al (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4):395–402
Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL (2010) H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene 29(37):5193–5203
Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J et al (2011) Loss of phasphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol 29(2):166–173
Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Aysegul SA, Hortobagyi GN, Yu D (2010) PTEN, PIK3CA, p-AKT, and p-p70S6K status—Association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol 177(4):1647–1656
Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, Sotiriou C, Fumagalli D, Jimenez J, Aura C et al (2015) PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol 56:2439
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6(2):117–127
Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM (2005) Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 65(2):473–482
Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL (2002) Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer 99(6):783–791
Mitra D, Brumlik M, Okamgba S, Zhu Y, Duplessis T, Parvani J, Lesko S, Brogi E, Jones F (2009) An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther 8(8):2152–2162
Rexer BN, Ham AJ, Rinehart C, Hill S, Granja-Ingram Nde M, Gonzålez-Angulo AM, Mills GB, Dave B, Chang JC, Liebler DC et al (2011) Phosphoproteomic mass spectrometry profiling links Src family kinases to escape from HER2 tyrosine kinase inhibition. Oncogene 30(40):4163–4174
Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z et al (2011) Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 17(4):461–469
Kwong KY, Hung MC (1998) Identification of a novel alternative splicing form of human HER2/neu proto-oncogene (ABSTRACT). Proc Am Assoc Cancer Res 39:205
Diermeier S, Horváth G, Knuechel-Clarke R, Hofstaedter F, Szöllösi J, Brockhoff G (2005) Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res 304:604–619
Motoyama AB, Hynes NE, Lane HA (2002) The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res 62(11):3151–3158
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445(7126):437–441
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J et al (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316(5827):1039–1043
Harris LN, You F, Schnitt SJ, Witkiewicz A, Lu X, Sgroi D, Ryan PD, Come SE, Burstein HJ, Lesnikoski B-A et al (2007) Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res 13(4):1198–1207
Huang X, Gao L, Wang S, McManaman JL, Thor AD, Yang X, Esteva FJ, Liu B (2010) Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to Herceptin. Cancer Res 70(3):1204–1214
Lu YH, Zi XL, Zhao YH, Mascarenhas D, Pollak M (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93(4):1852–1857
Nahta R, Yuan LX, Du Y, Esteva FJ (2007) Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther 6(2):667–674
Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J et al (2006) A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA 103(20):7795–7800
Wang Y-C, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, Botero MF, Healy NA, Hilsenbeck SG, Phillips GL et al (2011) Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers—role of estrogen receptor and HER2 reactivation. Breast Cancer Res 13(R121):1–19
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27(33):5529–5537
Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27(33):5538–5546
Burstein HJ, Cirrincione CT, Barry WT, Chew HK, Tolaney SM, Lake DE, Ma C, Blackwell KL, Winer EP, Hudis CA (2014) endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer—CALGB 40302 (Alliance). J Clin Oncol 32(35):3959–3966
Nahta R, Huan LXH, Zhang B, Kobayashi R, Esteva FJ (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65(23):11118–11128
Fox EM, Miller TW, Balko JM, Kuba MG, Sánchez V, Smith RA, Liu S, González-Angulo AM, Mills GB, Ye F et al (2011) A kinome-wide screen identifies the Insulin/IGF-1 receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res 71(21):6773–6784
Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H et al (1995) Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494
Lee AV, Weng CN, Jackson JG, Yee D (1997) Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J Endocrinol 152(1):39–47
Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A (2000) A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141(12):4503–4511
Jones HE, Goddard L, Gee JMW, Hiscox S, Rubini M, Barrow D, Knowlden JM, Williams S, Wakeling AE, Nicholson RI (2004) Insulin-like growth factor-1 receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 11(4):793–814
Balañá ME, Labriola L, Salatino M, Movsichoff F, Peters G, Charreau EH, Elizalde PV (2001) Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 19(1):34–47
Camirand A, Lu Y, Pollak M (2002) Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit 8(12):BR521–BR526
Fürsenberger G, Morant R, Senn HJ (2003) Insulin-like growth factors and breast cancer. Onkologie 26:290–294
Huynh H, Nickerson T, Pollak M, Yang X (1996) Regulation of insulin-like growth factor I receptor expression by the pure antiestrogen ICI 182780. Clin Cancer Res 2(12):2037–2042
Kahlert S, Neudling S, van Eickels M, Vetter H, Meyer R, Grohe C (2000) Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem 275(24):18447–18453
Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D (1999) Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol 13(5):787–796
Happerfield LC, Miles DW, Barnes DM, Thomsen LL, Smith P, Hanby A (1997) The localization of the insulin-like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J Pathol 183(4):412–417
Santen RJ, Jeng MH, Wang JP, Song R, Masamura S, McPherson R, Santner S, Yue W, Shim WS (2001) Adaptive hypersensivity to estradiol: potential mechanism for secondary hormonal responses in breast cancer patients. J Steroid Biochem Mol Biol 79((1–5 Speciall Issue SI)):115–125
Song RX, Barnes CJ, Zhang ZG, Bao YD, Kumar R, Santen RJ (2004) The role of Shc and insulin-like growth factor I receptor in mediating the translocation of estrogen receptor a to the plasma membrane. Proc Natl Acad Sci USA 101(7):2076–2081
Song RX, Santen RJ, Kumar R, Adam L, Jeng MH, Masamura S, Yue W (2002) Adaptive mechanisms induced by long-term estrogen deprivation in breast cancer cells. Mol Cell Endocrinol 193(1–2 Special Issue SI):29–42
Song RXD, McPherson RA, Adam L, Bao YD, Shupnik M, Kumar R, Santen RJ (2002) Linkage of rapid estrogen action to MAPK activation by ER alpha-Shc association and Shc pathway activation. Mol Endocrinol 16(1):116–127
Zhang ZG, Maier B, Santen RJ, Song RXD (2002) Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activatioin. Biochem Biophys Res Commun 294(5):926–933
Razandi M, Pedram A, Park ST, Levin ER (2003) Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278(4):2701–2712
Chakraborty AK, Liang K, DiGiovanna MP (2008) Co-targeting insulin-like growth factor I receptor and HER2: Dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res 68(5):1538–1545
Wang C-X, Koay DC, Edwards A, Lu Z, Mor G, Ocal IT, DiGiovanna MP (2005) In vitro and in vivo effects of combination of trastuzumab (Herceptin) and tamoxifen in breast cancer. Breast Cancer Res Treat 92(3):251–263
Chakraborty AK, Welsh A, DiGiovanna MP (2010) Co-targeting the insulin-like growth factor I receptor enhances growth-inhibitory and pro-apoptotic effects of anti-estrogens in human breast cancer cell lines. Breast Cancer Res Treat 120:327–335
Kaufman PA, Ferrero JM, Bourgeois H, Kennecke H, DeBoer R, Jacot W, McGreivy J, Suzuki S, Loh E, Robertson J (2010) A ramdomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC). Cancer Res 70(24):76s (ABSTRACT S71–74)
Robertson JFR, Ferrero J-M, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I et al (2013) Ganitumab with either exemestane or fulvestrant fo postmenopausal women with advanced, hormonereceptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 14:228–235
Haluska P, Bernath AM, Ballman KV, Dueck AC, Linden HM, Goetz MP, Northfelt DW, Hou X, Tenner KS, Tienchaiananda P et al (2014) Randomized phase II trial of capecitabine and lapatinib with or without cixutumumab in patients with HER2+ breast cancer previously treated with trastuzumab and an anthracycline and/or a taxane: NCCTG N0733 (Alliance). J Clin Oncol 32(supplement(5s)):Abstract 632
Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R et al (2010) Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): Rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther 9(10):2652–2664
R: A language and environment for statistical computing [http://www.R-project.org/]
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F et al (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10(6):515–527
Riedemann J, Takiguchi M, Sohall M, Macaulay VM (2007) The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun 355(3):707–714
Canonici A, Gijsen M, Mullooly M, Bennett R, Bouguern N, Pedersen K, O’Brien NA, Roxanis I, Li J-L, Bridge E et al (2013) Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 4(10):1592–1605
Wissner A, Mansour TS (2008) The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm Chem Life Sci 341:465–477
Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS et al (2005) Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 11:2063–2073
Browne BC, Eustace AJ, Kennedy S, O’Brien NA, Pedersen K, McDermott MSJ, Larkin A, Ballot J, Mahgoub T, Sclafani F et al (2012) Evaluation of IGF1R and phosphorylated IGF1R as targets in HER2-positive breast cancer cell lines and tumours. Breast Cancer Res Treat 137:717–727
Hou X, Huang F, Macedo LF, Harrington SC, Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM, Carboni JM et al (2011) Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res 71(24):7597–7607
Authors’ contributions
MPD designed and conceived of the project, analyzed and interpreted the data, and drafted the manuscript. AC designed and conducted all experiments, was responsible for all acquisition of data, participated in the analysis and interpretation of the data, participated in drafting and revising the manuscript, and gave final approval of the version to be published. CH performed the analyses and interpretation of drug interaction effects, participated in drafting and revising the manuscript, and gave final approval of the version to be published.
Funding
Connecticut Breast Health Initiative, and Pfizer Inc. The funding bodies had no role in the design of the study or the collection, analysis or interpretation of the data, or in the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial relationships with the funding sponsors. MPD has received royalties from DAKO and NeoMarkers, clinical trial funding from Genentech, a consulting fee from Merck, and payment for legal consulting from ImmunoGen.
Ethical standards
The experiments described here comply with current laws of the United States.
Rights and permissions
About this article
Cite this article
Chakraborty, A., Hatzis, C. & DiGiovanna, M.P. Co-targeting the HER and IGF/insulin receptor axis in breast cancer, with triple targeting with endocrine therapy for hormone-sensitive disease. Breast Cancer Res Treat 163, 37–50 (2017). https://doi.org/10.1007/s10549-017-4169-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4169-9