Abstract
Purpose
The Oncotype DX® Breast Recurrence Score™ (RS) assay is validated to predict breast cancer (BC) recurrence and adjuvant chemotherapy benefit in select patients with lymph node-positive (LN+), hormone receptor-positive (HR+), HER2-negative BC. We assessed 5-year BC-specific survival (BCSS) in LN+ patients with RS results in SEER databases.
Methods
In this population-based study, BC cases in SEER registries (diagnosed 2004–2013) were linked to RS results from assays performed by Genomic Health (2004–2014). The primary analysis included only patients (diagnosed 2004–2012) with LN+ (including micrometastases), HR+ (per SEER), and HER2-negative (per RT-PCR) primary invasive BC (N = 6768). BCSS, assessed by RS category and number of positive lymph nodes, was calculated using the actuarial method.
Results
The proportion of patients with RS results and LN+ disease (N = 8782) increased over time between 2004 and 2013, and decreased with increasing lymph node involvement from micrometastases to ≥4 lymph nodes. Five-year BCSS outcomes for those with RS < 18 ranged from 98.9% (95% CI 97.4–99.6) for those with micrometastases to 92.8% (95% CI 73.4–98.2) for those with ≥4 lymph nodes. Similar patterns were found for patients with RS 18–30 and RS ≥ 31. RS group was strongly predictive of BCSS among patients with micrometastases or up to three positive lymph nodes (p < 0.001).
Conclusions
Overall, 5-year BCSS is excellent for patients with RS < 18 and micrometastases, one or two positive lymph nodes, and worsens with additionally involved lymph nodes. Further analyses should account for treatment variables, and longitudinal updates will be important to better characterize utilization of Oncotype DX testing and long-term survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The 21-gene Oncotype DX® Breast Recurrence Score™ (RS) assay (Genomic Health, Inc., Redwood City, CA, USA) aids in adjuvant chemotherapy decision-making for those with early-stage hormone receptor-positive (HR+), HER2-negative breast cancer. The assay was initially validated to predict recurrence risk and chemotherapy benefit among those with lymph node-negative disease [1, 2]. In this context, the RS assay provides additional information to the chemotherapy decision-making process, and has been shown to impact chemotherapy recommendations [3–6], reduce overall chemotherapy overuse [7], and be cost-effective [8].
Following its commercial availability in 2004, the test became covered by insurers beginning in 2006 and has been incorporated into current clinical guidelines beginning in 2007 for use among those with lymph node-negative disease [9]. Retrospective evidence accrued thereafter suggested that the test is also predictive among select patients with minimal lymph node involvement (1–3 positive nodes) [10, 11]. RS results in select lymph node-positive (LN+) patients have been validated to be prognostic for 10-year distant recurrence and breast cancer-specific survival (BCSS) [10, 11] and predictive of chemotherapy benefit in prospectively designed studies of archival tissue [10]. As a result, the 2015 National Comprehensive Cancer Network Clinical Practice Guidelines in Breast Cancer have incorporated Oncotype DX testing into clinical guidelines for LN+ patients, specifically noting that Oncotype DX testing can be considered for patients with 1–3 involved ipsilateral axillary lymph nodes [12].
Currently, the RxPONDER trial is underway to evaluate the use of the 21-gene assay for breast cancer patients with 1–3 positive lymph nodes (HR+, HER2-negative), by randomizing research participants with a RS ≤ 25 to receive hormonal therapy alone or hormonal therapy with adjuvant chemotherapy [13]. Awaiting results from RxPONDER, limited information remains available about outcomes of contemporary patients with LN+ disease who were treated based on RS results. As such, the primary objective of this study was to assess 5-year BCSS among those with LN+ breast cancer who have oncotype DX RS results.
Methods
Data source
The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) breast cancer cases (diagnosed 2004–2012) were linked to 21-gene Oncotype DX Breast Recurrence Score assay results from the Genomic Health Clinical Laboratory (2004–2013) by Information Management Services (IMS, Inc.; Calverton, MD, USA). IMS released the de-identified, linked dataset to the study team for analyses following SEER approval.
Study population
The study population included men and women with estrogen receptor (ER)- and/or progesterone receptor (PR)-positive invasive breast cancer as defined by the SEER-reported ER and PR (positive or borderline) immunohistochemistry results. We include men because treatment guidelines for male breast cancer are similar to those for female breast cancer, and evidence indicates that the distribution of Recurrence Score results among men is similar to that among women [14]. We excluded those (1) with distant metastases, (2) affected by Hurricane Katrina (incident cases in Louisiana from July to December 2005), (3) with breast cancer diagnosis over the age of 99, (4) with zero years of survival time, (5) with autopsy data only, and (6) without a state death certificate. The cohort was stratified by lymph node status: lymph node-negative (N0, including isolated tumor cells), micrometastases only (N1mi), 1 positive lymph node (1LN), 2 positive lymph nodes (2LN), 3 positive lymph nodes (3LN), or 4–90 positive lymph nodes (≥4LN). We examined uptake of the 21-gene assay from 2004 to 2013 using these inclusion/exclusion criteria (n = 352,468).
For the remaining analyses, we further restricted the study population. We included only those who had a 21-gene assay Recurrence Score result within 12 months following breast cancer diagnosis among those diagnosed between 2004 and 2012. Furthermore, we excluded patients with HER2-positive disease. HER2 status was determined by the RT-PCR single-gene HER2 scores (≤11.5 = HER2-negative) performed by Genomic Health, Inc., as HER2 status was unavailable in SEER prior to 2010.
Measures
Uptake was defined as the proportion of patients in the study cohort who had a 21-gene assay ordered within 12 months following breast cancer diagnosis.
BCSS was calculated using cause of death data reported by the SEER registries, which link with state death certificates and the National Center for Health Statistics’ National Death Index [15]. These analyses utilized a variable derived by the SEER program that uses a mapping to dichotomize causes of death as breast cancer-specific or other-cause specific [16]. In the estimates of BCSS, follow-up is censored at the time of other-cause deaths or last known follow-up. In the estimates of other-cause survival, follow-up is censored at the time of breast cancer-specific death or last known follow-up. Overall survival was estimated by including all deaths as events regardless of cause.
Statistical analyses
For the 21-gene assay uptake evaluation, the proportion tested was calculated among those with HR+, non-metastatic, invasive breast cancer as the denominator. The proportions were calculated and plotted by year of breast cancer diagnosis (from 2004 to 2013). Descriptive statistics of the analytic sample were tested using ANOVA and Chi square to compare sample characteristics by number of positive lymph nodes. The survival analysis used the actuarial method using SAS version 9.4 (SAS Institute Inc.; Cary, NC) and was stratified by 21-gene assay RS cutpoints (RS < 18, RS 18–30 or RS ≥ 31) and by number of positive lymph nodes. A two-degrees-of-freedom log-rank test was used to determine if there was evidence of differences across the three categorical RS groups. Survival curves are presented through 5 years, but all observed events and follow-up were included for statistical testing purposes. Confidence intervals were computed using the log–log transformation. As a sensitivity analysis, we also examined BCSS by 21-gene assay RS cutpoints that correspond to those used in the RxPONDER trial (RS < 11, RS 11–25, RS ≥ 26) [13].
Exploratory analysis
Chemotherapy use among those with RS results was examined as an exploratory aim. Chemotherapy was assessed using SEER-reported data from 2004 to 2012. The proportion of those for whom the registries reported chemotherapy use (versus those reported as no or unknown) was plotted by the standard RS cutpoints (low < 18, intermediate 18–30 or high ≥ 31) and by continuous RS value (0–100). This aim was exploratory, as chemotherapy is under-reported in SEER [17], and as such the results must be interpreted with caution.
Results
Of the 352,468 HR+ and non-metastatic breast cancer cases, 74,207 patients had a 21-gene breast cancer assay ordered (Supplemental Fig. 1). Overall, the uptake of the 21-gene breast cancer assay increased over time (Fig. 1), with lower uptake among patients with greater lymph node involvement. For example, during 2004–2013, only 1.6% of those with ≥4LN received the 21-gene breast cancer assay compared with 21.6% of those with micrometastases. Uptake of the 21-gene breast cancer assay increased from 0.7% in 2004 to 44.4% in 2013 among those with micrometastases.
The proportion of individuals receiving Recurrence Score testing who had HR+, non-metastatic breast cancer, by year and number of positive lymph nodes. N0 lymph node-negative, N1mi micrometastases only, 1LN 1 positive lymph node, 2LN 2 positive lymph nodes, 3LN 3 positive lymph nodes, ≥4LN 4, or more positive lymph nodes
Patient characteristics of those with LN+ disease diagnosed between 2004 and 2012 who received a Recurrence Score result (n = 6768) varied by lymph node involvement. More specifically, patients with greater lymph node involvement tended to be older, to have tumors that were larger and higher in grade, to have higher mean RS results, and to live within a lower socioeconomic status (SES) area, compared with those with lower lymph node involvement (Table 1). There were no significant differences across lymph node status by race, year of diagnosis, or ER/PR status.
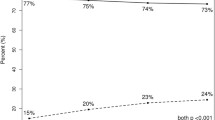
Chemotherapy reported as “yes” increased as lymph node involvement and RS value increased (Fig. 2). For example, among those with RS < 18, the registries reported 19% of patients with micrometastases as “yes” for chemotherapy use, compared with 56% of those with ≥4LN. Among those with ≥4LN, the proportion for whom the registries reported as “yes” for chemotherapy use increased from 56% among those with low RS results (RS < 18) to 77% among those with high RS results (RS ≥ 31). Reported chemotherapy was also modestly higher for patients with larger tumors or poorly differentiated tumors; however, age was the only variable with an effect of comparable magnitude to nodal status or RS results.
Chemotherapy use reported as “yes” versus “no/unknown,” by Recurrence Score results and number of positive lymph nodes. Overall chemotherapy use was reported as “yes” in 32.1% with N1mi, 34.6% with 1LN, and 43.9% with 2–3LN. For comparative purposes, chemotherapy use reported for lymph node-negative patients (N = 49,681), by Recurrence Score results, are shown in Supplemental Fig. 2
Five-year BCSS outcomes for those with RS < 18 ranged from 98.9% (95% CI 97.4–99.6) in those with micrometastases to 92.8% (95% CI 73.4–98.2) among those with ≥4LN. Similar patterns of worsening BCSS with increasing number of positive lymph nodes were found for those with RS results 18–30 and ≥31 (Table 2). The RS result was strongly predictive of BCSS among the group with micrometastases or 1–3LN, and the group with micrometastases or 1LN (both p < 0.001; Fig. 3). RS results were not significantly associated with other non-BCSS (Fig. 3). In a multivariable Cox model adjusting for grade, tumor size, age, and race, both the number of positive lymph nodes and the RS category remained strongly associated with BCSS (p < 0.001) (Supplemental Table 1).
Five-year breast cancer-specific survival (a, c, e) and other-cause (non-breast cancer-specific) survival (b, d, f), by Recurrence Score group among patients with micrometastases and 1 positive lymph node (N1mi, 1LN; N = 5440; a, b), patients with 2–3 positive lymph nodes (2–3LN; N = 1043; c, d), and patients with ≥4 positive lymph nodes (≥4LN; N = 285; e, f). For comparative purposes, 5-year BCSS and other-cause (non-BCSS) among patients with micrometastases up to three positive lymph nodes, by Recurrence Score group, are shown in Supplemental Fig. 3. Also, 5-year overall survival, by Recurrence Score group and number of positive lymph nodes, is shown in Supplemental Fig. 4
Discussion
Overall, 5-year BCSS is high in patients with RS < 18, particularly among those with micrometasteses and fewer positive lymph nodes. Five-year survival worsened both as the number of involved lymph nodes increased, and as RS result increased. Not all patients with LN+ disease had aggressive disease. Patients with RS < 18 had good survival and a low proportion for whom the registries reported as “yes” for chemotherapy use, suggesting that the addition of chemotherapy to adjuvant treatment in this RS group may have relatively little impact on BCSS. Furthermore, our findings support evidence that those with LN+ disease who receive 21-gene assay testing tend to be older and to have lower grade, smaller tumors, compared with those who do not receive such testing [18], and that rates of test uptake have increased over time among LN+ patients [19].
Future analyses that account for treatment are warranted; in particular, a comparison of survival among those who did and did not receive chemotherapy is of interest. Longitudinal data updates through the SEER and Genomic Health Clinical Laboratory linkage will allow for better characterization of how the 21-gene breast cancer assay is used, as well as survival outcomes among LN+ patients. Studying health services use of the 21-gene breast cancer assay and associated health outcomes in LN+ patients are particularly important, as our findings indicate increasing uptake of the assay.
Limitations
This study has several limitations. First, follow-up time has been relatively short to date; as such, additional analyses with longer follow-up are warranted. Of note, most chemotherapy benefit, if any, is observed in the first 5 years, and 5-year BCSS predicts 10- and 15-year BCSS [4]. Thus, study findings are informative for the decision to treat with chemotherapy and for longer-term survival outcomes. Second, future studies should incorporate treatment data into survival analyses; specifically, to examine survival by chemotherapy use and to account for surgery and/or radiation therapy. Chemotherapy use is known to be under-reported to SEER, and therefore such analyses were suboptimal in this study. Moreover, estimates of chemotherapy use should be interpreted with caution, as they likely under-report chemotherapy uptake. Third, the SEER registries do not collect information on distant recurrence, and this outcome could not be evaluated. Finally, we were unable to restrict the 21-gene assay uptake analyses by HER2-negative status in Fig. 1, as SEER did not begin collecting HER2 data until 2010. This may have led to an underestimate of 21-gene assay uptake prior to 2010 and to challenges to the comparison of survival between tested and untested patients.
Conclusions
This SEER study adds to the current understanding of survival outcomes among LN+ patients who receive the 21-gene breast cancer assay RS results. Our findings demonstrate high survival outcomes among those with low RS results, and lesser lymph node involvement. Future studies should continue to examine longer-term survival outcomes and incorporate treatment variables into analyses.
Abbreviations
- 1LN:
-
One positive lymph node
- 1–3LN:
-
One to three positive lymph nodes
- 2LN:
-
Two positive lymph nodes
- 2–3LN:
-
Two to three positive lymph nodes
- 3LN:
-
Three positive lymph nodes
- ≥4LN:
-
Four or more positive lymph nodes
- ANOVA:
-
Analysis of variance
- BC:
-
Breast cancer
- BCSS:
-
Breast cancer-specific survival
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR+:
-
Hormone receptor-positive (ER-positive, PR-positive, or both)
- LN+:
-
Lymph node-positive
- N1mi:
-
Micrometastases
- PR:
-
Progesterone receptor
- RS:
-
Recurrence Score®
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- RxPONDER:
-
Treatment (Rx) for positive node, endocrine-responsive breast cancer
- SD:
-
Standard deviation
- SEER:
-
Surveillance, epidemiology, and end results
- SES:
-
Socioeconomic status
References
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. doi:10.1056/NEJMoa041588
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. doi:10.1200/JCO.2005.04.7985
Albanell J, Gonzalez A, Ruiz-Borrego M, Alba E, Garcia-Saenz JA, Corominas JM, Burgues O, Furio V, Rojo A, Palacios J, Bermejo B, Martinez-Garcia M, Limon ML, Munoz AS, Martin M, Tusquets I, Rojo F, Colomer R, Faull I, Lluch A (2012) Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol 23(3):625–631. doi:10.1093/annonc/mdr278
Asad J, Jacobson AF, Estabrook A, Smith SR, Boolbol SK, Feldman SM, Osborne MP, Boachie-Adjei K, Twardzik W, Tartter PI (2008) Does Oncotype DX Recurrence Score affect the management of patients with early-stage breast cancer? Am J Surg 196(4):527–529. doi:10.1016/j.amjsurg.2008.06.021
Henry LR, Stojadinovic A, Swain SM, Prindiville S, Cordes R, Soballe PW (2009) The influence of a gene expression profile on breast cancer decisions. J Surg Oncol 99(6):319–323. doi:10.1002/jso.21244
Joh JE, Esposito NN, Kiluk JV, Laronga C, Lee MC, Loftus L, Soliman H, Boughey JC, Reynolds C, Lawton TJ, Acs PI, Gordan L, Acs G (2011) The effect of Oncotype DX Recurrence Score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. Oncologist 16(11):1520–1526. doi:10.1634/theoncologist.2011-0045
Dinan MA, Mi X, Reed SD, Lyman GH, Curtis LH (2015) Association between use of the 21-gene Recurrence Score assay and receipt of chemotherapy among medicare beneficiaries with early-stage breast cancer, 2005–2009. JAMA Oncol 1(8):1098–1109. doi:10.1001/jamaoncol.2015.2722
Reed SD, Dinan MA, Schulman KA, Lyman GH (2013) Cost-effectiveness of the 21-gene recurrence score assay in the context of multifactorial decision making to guide chemotherapy for early-stage breast cancer. Genet Med 15(3):203–211. doi:10.1038/gim.2012.119
Trosman JR, Van Bebber SL, Phillips KA (2010) Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract 6(5):238–242. doi:10.1200/JOP.000075
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North America (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65. doi:10.1016/S1470-2045(09)70314-6
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834. doi:10.1200/JCO.2009.24.4798
National Comprehensive Cancer Network (2015) NCCN Clinical practice guidelines in oncology: breast cancer. Invasive breast cancer. https://www.nccn.org/
Wong WB, Ramsey SD, Barlow WE, Garrison LP Jr, Veenstra DL (2012) The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007). Contemp Clin Trials 33(6):1117–1123. doi:10.1016/j.cct.2012.08.006
Grenader T, Yerushalmi R, Tokar M, Fried G, Kaufman B, Peretz T, Geffen DB (2014) The 21-gene recurrence score assay (Oncotype DX) in estrogen receptor-positive male breast cancer: experience in an Israeli cohort. Oncology 87(1):1–6. doi:10.1159/000360793
National Center for Health Statistics (2016) http://www.cdc.gov/nchs/index.htm. Accessed 6 July 2016
Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102(20):1584–1598. doi:10.1093/jnci/djq366
Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL (2016) Comparison of SEER treatment data with medicare claims. Med Care 54(9):e55–e64. doi:10.1097/MLR.0000000000000073
Petkov V, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, Glaser SL, Hernandez BY, Lynch CF, Mueller L, Schwartz AG, Schwartz SM, Stroup A, Sweeney C, Tucker TC, Ward KC, Wiggins C, Wu X, Penberthy L, Shak S (2016) Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer 2:16017. doi:10.1038/npjbcancer.2016.17
Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Carey LA, Troester MA, Wheeler SB (2016) Racial variation in the uptake of oncotype DX testing for early-stage breast cancer. J Clin Oncol 34(2):130–138. doi:10.1200/JCO.2015.63.2489
Acknowledgements
We acknowledge Anna Lau for medical writing and editorial assistance. The ideas and opinions expressed herein are those of the author(s) and endorsement by any State, Department of Public Health, the National Cancer Institute, the Centers for Disease Control and Prevention, or their Contractors and Subcontractors is not intended nor should be inferred. The Surveillance, Epidemiology and End Results (SEER) Program is funded by the National Cancer Institute (NCI). Genomic Health performed the work to electronically submit the Recurrence Score results, but provided no funding for this study. We acknowledge the SEER registries for collecting the SEER data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Roberts and Dr. Petkov declare no conflicts of interest. Mr. Dave Miller and Dr. Stephen Shak are employed by and have stock ownership in Genomic Health Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberts, M.C., Miller, D.P., Shak, S. et al. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Cancer Res Treat 163, 303–310 (2017). https://doi.org/10.1007/s10549-017-4162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4162-3