Abstract
Purpose
To examine whether baseline sleep duration or changes in sleep duration are associated with breast cancer prognosis among early-stage breast cancer survivors in the multi-center Women’s Healthy Eating and Living Study.
Methods
Data were collected from 1995 to 2010. Analysis included 3047 women. Sleep duration was self-reported at baseline and follow-up intervals. Cox proportional hazard models were used to investigate whether baseline sleep duration was associated with breast cancer recurrence, breast cancer-specific mortality, and all-cause mortality. Time-varying models investigated whether changes in sleep duration were associated with breast cancer prognosis.
Results
Compared to women who slept 7–8 h/night at baseline, sleeping ≥9 h/night was associated with a 48% increased risk of breast cancer recurrence (Hazard ratio [HR] 1.48, 95% Confidence interval [CI] 1.01, 2.00), a 52% increased risk of breast cancer-specific mortality (HR 1.52, 95% CI 1.09, 2.13), and a 43% greater risk of all-cause mortality (HR 1.43, 95% CI 1.07, 1.92). Time-varying models showed analogous increased risk in those who inconsistently slept ≥9 h/night (all P < 0.05), but not in those who consistently slept ≥9 h/night.
Conclusions
Consistent long or short sleep, which may reflect inter-individual variability in the need for sleep, does not appear to influence prognosis among early-stage breast cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 3 million women in the US have been diagnosed with and treated for breast cancer [1]. Up to 70% of these breast cancer survivors report sleep problems which can include delayed sleep onset, difficulty in maintaining sleep, and early morning waking, which can influence sleep-related behaviors and overall sleep time [2, 3]. Given the high prevalence of sleep-related problems in this large population of women, research efforts are needed to understand the role of sleep duration and breast cancer prognosis.
Considerable epidemiologic evidence indicates that sleep is intimately linked to health outcomes, with studies documenting that both short and long sleep are associated with early mortality in the general population [4–6]. A number of mechanisms have been postulated to explain the association between sleep duration and breast cancer, including alterations in metabolism, the circadian system, and immune function [7–14]. For example, sleep deprivation has been shown to result in metabolic changes that may predispose an individual to breast cancer, such as altered endocrine function and glucoregulation [7], and increased inflammation [8, 9]. There is also evidence that alterations in sleep influence the alignment between the sleep/wake cycle and the endogenous circadian system, resulting in circadian misalignment [10]. Links between sleep and the circadian system are particularly relevant to breast cancer, given the considerable epidemiologic evidence that chronic circadian misalignment is associated with breast cancer [11, 12]. Finally, sleep may influence susceptibility to cancer by regulating melatonin, a hormone that has anti-cell proliferative activity [13].
The influence of sleep on breast cancer prognosis is less clear. A recent study among breast cancer survivors in the Women’s Health Initiative found short sleep (sleeping ≤5 h/night) before diagnosis to be associated with poorer breast cancer survival compared to women who slept 7–8 h per night [15]. Many patients develop new sleep problems after their diagnosis of breast cancer [2, 16] and thus post-diagnosis sleep duration may have additional implications for future morbidity and mortality among breast cancer survivors. Factors contributing to the particularly high rates of sleep problems among breast cancer survivors may include the occurrence or exacerbation of menopausal symptoms (e.g., hot flashes) caused by chemotherapy or endocrine therapy [17]. Other factors contributing to sleep problems among breast cancer survivors may stem from the increased stress or pain as a result of the cancer diagnosis and treatments [2]. We are only aware of a few studies that have examined the influence of post-diagnosis sleep and sleep-related variables on breast cancer prognosis [18, 19], but they were conducted in populations of metastatic patients. Therefore, it is unclear if findings are relevant to survivors of early-stage breast cancer, who are considered cancer free after treatment.
This analysis used data from the Women’s Healthy Eating and Living (WHEL) study to investigate whether post-diagnosis sleep duration predicts breast cancer recurrence, breast cancer-specific mortality, and all-cause mortality among survivors of early-stage breast cancer. Additionally, given the evidence that sleeping habits are not static and may change across the post-diagnosis interval among early-stage breast cancer patients [3], we also examined associations between changes in sleep duration over study follow-up and breast cancer prognosis. We hypothesize that models of sleep duration change will provide a unique and more informative estimation of associations between post-diagnosis sleep duration and breast cancer prognosis.
Methods
Details of eligibility criteria, recruitment, data collection, and outcome ascertainment have been reported previously [20, 21] and are briefly summarized here.
Participants
Participants were breast cancer survivors from the WHEL Study. The WHEL Study was a multisite randomized trial that enrolled 3088 women within 4 years of diagnosis of early-stage breast cancer (mean = 2.0 years) to test the hypothesis that a diet rich in vegetables, fruit, and fiber and low in fat could reduce breast cancer recurrence and mortality [20]. There was no intervention effect on breast cancer recurrence or mortality after an average of 7.3 years of follow-up [21]. Therefore, this analysis treated women enrolled in the WHEL Study as a single cohort. Institutional review boards at the University of California, San Diego and 6 other clinical sites approved the study protocol and procedures, and all participants provided written informed consent.
Sleep
Sleep duration was assessed by asking participants “About how many hours of sleep did you get on a typical night during the past 4 weeks?” Response options were: 5 or less, 6, 7, 8, 9, or 10 or more hours per night. This analysis used sleep duration assessments collected at baseline, year 1, years 2, or 3 (either-or), and year 4. Approximately 56% of the sample had sleep records available for all 4 time points; 18% had sleep records available at 3 time points; and 26% had sleep records available at 2 or fewer time points. Insomnia was assessed using the 5-item WHI Insomnia Rating Scale (WHI-IRS) [22]. An overall insomnia index score was calculated using the standard algorithm of the average scores for trouble falling asleep, waking several times, waking early, trouble resuming sleep, and overall sleep quality [22]. Scores ≥9 indicate clinically significant insomnia with acceptable sensitivity [23]. The WHI-IRS has high internal consistency (Cronbach’s alpha = 0.79) [23] and test–retest (r = 0.84 for 8–14 days) reliability [23], and adequate construct validity as demonstrated by its ability to detect sleep disturbances in groups of women known to differ in the severity of vasomotor symptoms [24].
Other assessments
Medical records were abstracted at the study baseline for clinical information about the initial breast cancer diagnosis and treatment, including stage, grade, hormone receptor status, and use of radiotherapy, chemotherapy, or endocrine therapy (e.g., tamoxifen). Demographic characteristics of participants were also self-reported at baseline. Height, weight, and co-morbidity data used in this analysis were measured at the baseline, year 1, years 2, or 3, and year 4 clinic visits. Height and weight were used to calculate body mass index (BMI [kg/m2]). Co-morbidities were assessed using a self-administered questionnaire regarding whether participants were currently being treated for a variety of diseases and conditions [25]. Participants were asked about cardiovascular disease (angina, peripheral arterial disease, other heart conditions), diabetes, gallbladder/kidney/pancreas disease (gallbladder disease, kidney/bladder stones, pancreatitis), gastrointestinal disorders (ulcers, diverticulitis, colitis, intestinal removal, irritable bowel syndrome, and malabsorption syndrome), arthritis, and osteoporosis. The specific diseases were combined into general systems (such as cardiovascular disease) to mirror the well-validated Charlson index [26]. Patients were also asked if they were being treated for hypertension and high blood cholesterol. The number of diseases which participants were currently being treated for was summed at each time interval to calculate the total number of co-morbid conditions. Quality of life was assessed using the MOS 36-item Health Survey (SF-36) [27], which summarizes four physical and four mental health subscales. The questionnaire has been used in diverse populations, including women with breast cancer [28], and has shown to be reliable (Cronbach’s alpha = 0.75–0.91) [29] with good construct validity [30].
Follow-up and study outcomes
The outcomes of this analysis were breast cancer recurrence, breast cancer-specific mortality, and all-cause mortality. Breast cancer recurrence was ascertained through active surveillance (semi-annual or annual telephone calls) with all reported events confirmed by medical record review. Breast cancer recurrence was defined as the combined outcome of either an invasive breast cancer recurrence or a new primary invasive breast cancer. The breast cancer recurrence-free interval was defined as the time from the original breast cancer diagnosis to the development of a new breast cancer event or end of study follow-up. Follow-up time for breast cancer recurrence was censored at the last documented staff contact date or at study completion (June, 2010).
Information on breast cancer-specific mortality and all-cause mortality was acquired by periodic review of the Social Security Death Index for a mean follow-up of 13.5 years (Standard deviation [SD] = 3.76). Cause of death was obtained from International Classification of Diseases, 9th Revision (ICD-9) codes obtained from death certificates of each decedent. Survival time was defined as the time from breast cancer diagnosis to death, or the most recently available review of the Social Security Death Index.
Statistical analysis
Given the evidence that sleep duration appears to have a U-shaped association with mortality [5, 6], we modeled sleep duration as a categorical variable. We collapsed the ≤5 and 6 h categories, the 7 and 8 h categories, and 9 and ≥10 h categories due to small cell sizes. Therefore, the final sleep duration categories were ≤6 h/night (referred to as ‘short sleep’), 7–8 h/night (referred to as ‘middle sleep’), and ≥9 h/night (referred to as ‘long sleep’). It is notable that the ‘middle sleep’ category is consistent with the National Sleep Foundation’s recommended sleep duration for appropriate sleep (7–9 h for adults, and 7–8 h for older adults [≥65 years]) [31].
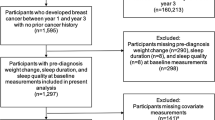
Attributes of the study sample were compared across baseline sleep duration categories using analysis of variance and Kruskal–Wallis tests for continuous variables and Chi-square tests for categorical variables. Ninety-four participants in the analytic sample were missing a baseline sleep assessment and so the first available assessment was used as baseline for these subjects. An additional 41 participants had no sleep records available and were excluded from this analysis.
Cox proportional hazards regression models were used to evaluate the association of baseline sleep duration categories with breast cancer recurrence, breast cancer-specific mortality, and all-cause mortality. Models were delayed entry to account for the varying hazards associated with different intervals of time between breast cancer diagnosis and enrollment in the WHEL study. Covariates selected a priori included age, stage, grade, race/ethnicity, intervention group, study site, BMI, and number of co-morbidities. Given that co-morbidity data were missing for n = 506 participants, we treated the number of co-morbidities as a categorical variable and included missing as a category. Human epidermal growth factor receptor 2 (Her-2) status testing was not a standard of care procedure at the time of WHEL Study enrollment, and therefore it was not included as a covariate in this analysis. However, we did consider adjustment for other tumor characteristics and treatment variables with potential links to sleep and/or breast cancer prognosis, including estrogen and progesterone receptor status, anti-estrogen use, radiotherapy and chemotherapy use, and menopausal status at diagnosis. We also considered adjustment for a wide range of lifestyle and behavioral characteristics, including alcohol intake, smoking status, energy intake, physical activity, Women’s Health Initiative insomnia rating scale (WHI-IRS) score, as well as the MOS 36-Item Short-Form Health Survey physical and mental health subscale scores. Because the addition of these variables to the models did not change the hazard ratios by more than 10%, they were not included in final models. Given the high prevalence of insomnia observed for women in this cohort, exploratory models were run to examine whether WHI-IRS scores predicted breast cancer prognosis. These WHI-IRS models adjusted for the same covariates as the sleep duration models described above.
Delayed entry Cox proportional hazards regression models were used to evaluate associations between breast cancer prognosis and sleep duration change categories, modeled as a time-varying covariate using methods outlined by Anderson and Gill [32], across baseline, year 1, years 2, or 3 (either-or), and year 4. Sleep duration change categories included consistent short (≤6 h/night), consistent middle (7–8 h/night), consistent long (≥9 h/night), inconsistently long, and inconsistently short. The inconsistently long sleep duration change category reflects longitudinal transitions from middle to long sleep duration categories (or vice versa) over time. The inconsistently short sleep duration change category reflects longitudinal changes from middle to short sleep duration categories (or vice versa) over time. Forty-five women transitioned between short and long sleep categories and were categorized as inconsistently long sleep duration, and sensitivity analyses examined whether results change if these women are removed from the sample. Sleep duration change models controlled for the same covariates as the single time-point Cox models described above, with the exception that the models adjusted for the total number of sleep assessments (recorded for each participant) to minimize compliance bias. The number of sleep assessments were updated at each time point and modeled as a time-varying covariate. In addition, BMI and number of co-morbidities were modeled as time-varying covariates. It is also notable that we conducted the same confounder assessment as single time-point models described previously. However, we also assessed the influence of the following time-varying covariates on the hazard ratios: alcohol intake, smoking status, energy intake, physical activity, WHI insomnia rating scale score, and SF-36 physical and mental health subscale scores [27]. The addition of these time-invariant and/or time-varying covariates did not change the hazard ratios by more than 10%, so they were not included in final models. The proportional hazards assumption was examined and satisfied in all Cox models by testing the significance of the product terms for the independent variable of interest and log time. All statistical tests were two sided with alpha set at P < 0.05 and all analyses were conducted using SAS version 9.4 (Cary NC).
Results
The study sample consisted of 3047 breast cancer survivors with a mean (SD) age of 52.8 (9.0) years and a BMI of 27.3 (6.1). A total of 2597 (85.2%) were white and 1651 (54.2%) were college educated. Participants had a median (IQR) insomnia rating scale score of 7.0 (7.0) (range = 0–20 [scores ≥ 9 indicate insomnia]). 46% of women had insomnia rating scale scores indicative of insomnia on 1 or more of her sleep questionnaires. Sleep duration categories were significantly associated with race/ethnicity, education, insomnia rating scale score, co-morbidities, physical activity, and BMI. Specifically, among white and non-white participants, roughly 6% were long sleepers, whereas the proportion of short sleepers was less than 30% among whites but close to 50% among non-whites. A higher proportion of college educated women were middle sleepers compared to women who did not complete college, whereas a greater proportion of non-college educated women were short sleepers. Also, a greater proportion of women without co-morbidities were middle sleepers, compared to women with 1, 2, or 3 or more co-morbidities. Women in the middle and long sleep duration categories had lower insomnia rating scale scores compared to women in the short sleep duration category. Healthy lifestyle behavior such as physical activity and BMI appeared to have a U-shaped association with sleep duration categories, with lower levels of physical activity and higher BMI in the long and short sleep duration categories (Table 1).
In baseline Cox proportional hazards regression models, long sleep duration (9 or more hours per night) was associated with a 48% higher hazard for breast cancer recurrence, compared to middle sleep duration (7–8 h per night) (Hazard ratio [HR] 1.48 95% Confidence interval [CI] 1.01, 2.00) (Table 2). Long sleep duration was also associated with a 52% higher hazard for breast cancer-specific mortality (HR 1.52 95% CI 1.09, 2.13), and a 43% higher hazard for all-cause mortality (HR 1.43 95% CI 1.07, 1.92). Short sleep duration was not significantly associated with breast cancer prognosis. Finally, in exploratory models of WHI-IRS and breast cancer prognosis, WHI-IRS scores were not associated with breast cancer recurrence, mortality, or breast cancer-specific mortality (all p > 0.05; data not shown).
Table 3 provides the results from a time-varying analysis of sleep duration change categories with breast cancer prognosis. Inconsistently long sleep duration over the study’s follow-up was associated with a 60% higher hazard for breast cancer recurrence, compared to consistent middle sleep duration (HR 1.60 95% CI 1.18, 2.18). Inconsistently long sleep duration was also associated with a 70% higher hazard for breast cancer-specific mortality (HR 1.70 95% CI 1.23, 2.36), and a 47% higher hazard for all-cause mortality (HR 1.47 95% CI 1.12, 1.93). Inconsistently short sleep was also associated with a 21% reduced risk of all-cause mortality (HR 0.79 95% CI 0.63, 0.98). However, inconsistently short sleep was not associated with risk of breast cancer recurrence or breast cancer-specific mortality. Consistent long or short sleep was not associated with breast cancer prognosis.
Sensitivity analyses confirmed that results of these analyses do not change after removing (1) the 45 women who transitioned between short and long sleep categories, (2) the 94 participants missing baseline sleep data, or (3) participants with only one sleep record (n = 432).
Discussion
These data suggest that when changes over time are not considered, sleeping 9 or more hours per night is associated with an increased risk of breast cancer recurrence, and increased risks for both breast cancer-specific mortality and all-cause mortality. However, the current study’s robust, time-varying approach to modeling sleep duration change indicates that consistently sleeping 9 or more hours per night does not increase risk for poor breast cancer prognosis. Rather, this study’s time-varying models indicate that women with inconsistent sleeping patterns—particularly transitions from middle to long (or vice versa)—may be associated with an increased risk of breast cancer recurrence, breast cancer-specific mortality, and all-cause mortality. Taken together, these findings suggest that single time-point models of sleep duration and breast cancer prognosis provide an incomplete picture of the associations between these parameters, which is a novel contribution to the literature. Importantly, this investigation underscores the need to study sleep patterns longitudinally.
Our finding that women who habitually reported 9 or more hours of sleep per night were not at an increased risk for poor breast cancer prognosis is consistent with the hypothesis that there may be differences in individuals’ need for sleep [33, 34]. In other words, some people may be programmed to need more (or less) sleep than others. For example, in a laboratory study among 21 healthy adults, Tucker and colleagues used polysomnography to explore inter-individual differences in sleep under a variety of controlled conditions [33]. The authors found that almost all of the sleep variables examined in their study demonstrated systematic inter-individual “trait-like” variability, and sleep duration was one of the key trait-like dimensions that sleep variance clustered around. Other laboratory studies have observed differences in components of the circadian pacemaker between habitual long and short sleepers. For example, data from an experimental study among 10 habitual long sleepers (sleep >9 h) and 14 habitual short sleepers (sleep <6 h) indicate that long sleepers tend to have a longer “biological night,” as characterized by a longer duration of high melatonin levels, low body temperature, and interval of increasing cortisol [34]. Such findings build on the evidence that what is experienced as ‘normal sleep’ may vary considerably across individuals.
Our finding that inconsistently sleeping 9 or more hours over study follow-up was associated with worse breast cancer prognosis could be explained by changes in lifestyle behaviors or health status. However, we found the effects of sleep duration change patterns on breast cancer prognosis to be independent of changes in lifestyle factors, physical and mental health, as well as co-morbidity status. It is also possible that changes in sleep duration reflect some other changes in psychosocial or health-related factors among women in the sample. For example, studies have demonstrated that exogenous administration of inflammatory cytokines is associated with somnolence and increased sleep [35–37]. Therefore, increased sleep duration may be a symptom of other physiologic processes in the body associated with poor breast cancer prognosis, such as systemic inflammation [38]. However, it is also notable that sleep has been shown to induce changes in metabolic inflammatory cytokine concentrations, which make it difficult to make inferences about the direction of causality. In an exploratory pilot trial among 14 healthy women by Reynold et al., a 3 h increase in time spent in bed resulted in a 2-fold increase in Interleukin-6 concentrations, whereas no changes were observed in women randomized to the comparison condition [39]. Finally, it is important to note that sleep patterns are often correlated with depressive symptoms in breast cancer survivors [40], which have been associated with shorter breast cancer survival [41, 42]. Among breast cancer patients, depression has also been associated with risk factors for poor breast cancer prognosis including endocrine and metabolic dysfunction [43, 44]. Therefore, it is possible that changes in depression status could explain some or all of the association between changes in sleep duration and breast cancer prognosis.
This analysis is limited by the use of self-reported sleep duration data, which is subject to error and may reflect the participant’s belief that a particular number of hours of sleep are desirable. We also acknowledge that other components of sleep quality not assessed in this study (such as subjective sleep restfulness, objective measures of time spent in rapid eye movement sleep, or sleep onset latency) may be important clinical variables to consider in the studies of sleep and breast cancer. Breast cancer survivors in the WHEL Study were also enrolled up to 4 years post-diagnosis (mean = 2.0 years); therefore, our findings may not be generalizable to patients who are newly diagnosed with breast cancer. Strengths of this study include the use of multiple modeling strategies to test our study hypothesis. This analysis also leveraged sleep duration data collected at multiple time points, which enabled us to conduct a time-varying analysis. Such time-varying analyses are infrequently conducted in the studies of sleep and cancer outcomes. Finally, we rigorously evaluated potential time-invariant and time-varying confounders.
In conclusion, our investigation indicates that habitually sleeping 9 or more or 6 or fewer h per night are not risk factors for poor breast cancer outcomes, highlighting the fact that associations between sleep duration and breast cancer prognosis are complex and may not be accurately portrayed by static (single time-point) models. Future prospective studies with sleep collected at multiple time points should consider using longitudinal models to investigate associations between sleep duration and breast cancer outcomes. Such studies could clarify associations between sleep duration patterns and breast cancer prognosis.
References
Howlander N, Noone A, Krapacho M, Miller D, Boship K, Alterkruse SF, C.L. K, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, E.J. F, Cronin KA SEER Cancer Statistics Revier, 1975–2013, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2013/. Based on November 2015 SEER data submission, posted to the SEER web site, April 2016
Bower JE (2008) Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol 26(5):768–777. doi:10.1200/JCO.2007.14.3248
Alfano CM, Lichstein KL, Vander Wal GS, Smith AW, Reeve BB, McTiernan A, Bernstein L, Baumgartner KB, Ballard-Barbash R (2011) Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat 130(1):243–254. doi:10.1007/s10549-011-1530-2
Tamakoshi A, Ohno Y, Group JS (2004) Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep 27(1):51–54
Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB (2004) A prospective study of sleep duration and mortality risk in women. Sleep 27(3):440–444
Hublin C, Partinen M, Koskenvuo M, Kaprio J (2007) Sleep and mortality: a population-based 22-year follow-up study. Sleep 30(10):1245–1253
Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354(9188):1435–1439. doi:10.1016/S0140-6736(99)01376-8
Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP (2004) Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89(5):2119–2126. doi:10.1210/jc.2003-031562
Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM (2004) Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43(4):678–683. doi:10.1016/j.jacc.2003.07.050
Morris CJ, Aeschbach D, Scheer FA (2012) Circadian system, sleep and endocrinology. Mol Cell Endocrinol 349(1):91–104. doi:10.1016/j.mce.2011.09.003
Schernhammer ES, Kroenke CH, Laden F, Hankinson SE (2006) Night work and risk of breast cancer. Epidemiology 17(1):108–111
Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES (2005) Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 41(13):2023–2032. doi:10.1016/j.ejca.2005.05.010
Vijayalaxmi Thomas CR Jr, Reiter RJ, Herman TS (2002) Melatonin: from basic research to cancer treatment clinics. J Clin Oncol 20(10):2575–2601. doi:10.1200/JCO.2002.11.004
Dimitrov S, Lange T, Nohroudi K, Born J (2007) Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 30(4):401–411
Phipps AI, Bhatti P, Neuhouser ML, Chen C, Crane TE, Kroenke CH, Ochs-Balcom H, Rissling M, Snively BM, Stefanick ML, Treggiari MM, Watson NF (2016) Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med 12(4):495–503. doi:10.5664/jcsm.5674
Costa AR, Fontes F, Pereira S, Goncalves M, Azevedo A, Lunet N (2014) Impact of breast cancer treatments on sleep disturbances—a systematic review. Breast 23(6):697–709. doi:10.1016/j.breast.2014.09.003
Savard J, Davidson JR, Ivers H, Quesnel C, Rioux D, Dupere V, Lasnier M, Simard S, Morin CM (2004) The association between nocturnal hot flashes and sleep in breast cancer survivors. J Pain Symptom Manag 27(6):513–522. doi:10.1016/j.jpainsymman.2003.10.013
Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D (2014) Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 37(5):837–842. doi:10.5665/sleep.3642
Hahm BJ, Jo B, Dhabhar FS, Palesh O, Aldridge-Gerry A, Bajestan SN, Neri E, Nouriani B, Spiegel D, Zeitzer JM (2014) Bedtime misalignment and progression of breast cancer. Chronobiol Int 31(2):214–221. doi:10.3109/07420528.2013.842575
Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, Haan M, Hollenbach KA, Jones L, Marshall JR, Ritenbaugh C, Stefanick ML, Thomson C, Wasserman L, Natarajan L, Thomas RG, Gilpin EA, Women’s Healthy E, Living study g (2002) A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials 23(6):728–756
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, Faerber S, Gold EB, Hajek RA, Hollenbach K, Jones LA, Karanja N, Madlensky L, Marshall J, Newman VA, Ritenbaugh C, Thomson CA, Wasserman L, Stefanick ML (2007) Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 298(3):289–298. doi:10.1001/jama.298.3.289
Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA (2003) Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess 15(2):123–136
Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, Shumaker SA (2003) Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess 15(2):137–148
Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA (2005) Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med 67(1):98–104. doi:10.1097/01.psy.0000151743.58067.f0
Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, Laughlin GA, Erickson K, Thomson CA, Bardwell WA, Hajek RA, Pierce JP (2010) Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat 122(3):859–865. doi:10.1007/s10549-010-0732-3
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6):473–483
Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA (2003) Health-related quality-of-life measurement in randomized clinical trials in breast cancer–taking stock. J Natl Cancer Inst 95(4):263–281
Kosinski M, Keller SD, Hatoum HT, Kong SX, Ware JE, Jr (1999) The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: tests of data quality, scaling assumptions and score reliability. Medical care 37(5 Suppl):MS10–MS22
Ware JE Jr, Gandek B (1998) Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol 51(11):903–912
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Hillard PJA, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC (2015) National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health 1(14):233–243. doi:10.1016/j.sleh.2015.10.004
Anderson PK, Gill RD (1982) Cox’s regression model for counting processes: a large sample study. Ann Stat 10(4):1100–1120. doi:10.1214/aos/1176345976
Tucker AM, Dinges DF, Van Dongen HP (2007) Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res 16(2):170–180. doi:10.1111/j.1365-2869.2007.00594.x
Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA (2003) A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab 88(1):26–30. doi:10.1210/jc.2002-020827
Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM (2011) Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med 7(5 Suppl):S38–S42. doi:10.5664/JCSM.1360
Krueger JM (2008) The role of cytokines in sleep regulation. Curr Pharm Des 14(32):3408–3416
Krueger JM, Takahashi ST, Kapas L, Bredow S, Roky R, Fang J, Floyd R, Renegar KB, Guha-Thakurta N, Novitsky S, Obar FJ (1995) Cytokines in sleep regulation. Adv Neuroimmunol 5:171–188
Villasenor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, Patterson RE (2014) Postdiagnosis C-reactive protein and breast cancer survivorship: findings from the WHEL study. Cancer Epidemiol Biomark Prev 23(1):189–199. doi:10.1158/1055-9965.EPI-13-0852
Reynold AM, Bowles ER, Saxena A, Fayad R, Youngstedt SD (2014) Negative Effects of Time in Bed Extension: a Pilot Study. J Sleep Med Disord 1(1):1
Bardwell WA, Natarajan L, Dimsdale JE, Rock CL, Mortimer JE, Hollenbach K, Pierce JP (2006) Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. J Clin Oncol 24(16):2420–2427. doi:10.1200/JCO.2005.02.0081
Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D (2011) Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 29(4):413–420. doi:10.1200/JCO.2010.28.4455
Suppli NP, Johansen C, Kessing LV, Toender A, Kroman N, Ewertz M, Dalton SO (2016) Survival after early-stage breast cancer of women previously treated for depression: a nationwide Danish cohort study. J Clin Oncol. doi:10.1200/JCO.2016.68.8358
Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB (2001) Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 158(8):1252–1257. doi:10.1176/appi.ajp.158.8.1252
Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D (2009) Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun 23(8):1148–1155. doi:10.1016/j.bbi.2009.07.007
Funding
Dr. Marinac was supported by the National Cancer Institute of the National Institutes of Health under award number F31 CA183125. Research support was also provided by funding from the National Cancer Institute under award numbers U54 CA155435, and R01 CA166293. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or conflicts of interest to disclose.
Ethical Approval
All procedures performed involving human participants were in accordance with the ethical standards of the University of California, San Diego and 6 other clinical sites, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Marinac, C.R., Nelson, S.H., Flatt, S.W. et al. Sleep duration and breast cancer prognosis: perspectives from the Women’s Healthy Eating and Living Study. Breast Cancer Res Treat 162, 581–589 (2017). https://doi.org/10.1007/s10549-017-4140-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4140-9