Abstract
Patient navigation is emerging as a standard in breast cancer care delivery, yet multi-site data on the impact of navigation at reducing delays along the continuum of care are lacking. The purpose of this study was to determine the effect of navigation on reaching diagnostic resolution at specific time points after an abnormal breast cancer screening test among a national sample. A prospective meta-analysis estimated the adjusted odds of achieving timely diagnostic resolution at 60, 180, and 365 days. Exploratory analyses were conducted on the pooled sample to identify which groups had the most benefit from navigation. Clinics from six medical centers serving vulnerable populations participated in the Patient Navigation Research Program. Women with an abnormal breast cancer screening test between 2007 and 2009 were included and received the patient navigation intervention or usual care. Patient navigators worked with patients and their care providers to address patient-specific barriers to care to prevent delays in diagnosis. A total of 4675 participants included predominantly racial/ethnic minorities (74 %) with public insurance (40 %) or no insurance (31 %). At 60 days and 180 days, there was no statistically significant effect of navigation on achieving timely diagnostic care, but a benefit of navigation was seen at 365 days (aOR 2.12, CI 1.36–3.29). We found an equal benefit of navigation across all groups, regardless of race/ethnicity, language, insurance status, and type of screening abnormality. Patient navigation resulted in more timely diagnostic resolution at 365 days among a diverse group of minority, low-income women with breast cancer screening abnormalities.
Trial registrations clinicaltrials.gov Identifiers: NCT00613275, NCT00496678, NCT00375024, NCT01569672.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inequities in breast cancer mortality persist, with recent evidence suggesting that they are worsening for certain vulnerable populations [1–4]. Delays in delivering timely breast cancer care lead to more advanced stage at diagnosis and ultimately more breast cancer deaths for low-income minority communities [3–7]. While the interplay of race, poverty, and other socioeconomic indicators on cancer outcomes is complex, the literature clearly documents the impact that barriers to accessing cancer care has on these patient’s outcomes [7–10]. The presence of patient-identified barriers to health care leads to delays in care and is increasingly scrutinized as a source of poor survival for many vulnerable populations [2, 4–6, 11–16], while large population-based studies report that equal high-quality treatment can significantly reduce disparities in mortality [2–17].
Patient navigation represents a promising practice to reduce disparities in cancer care delivery by intervening to address barriers to timely care. Navigation is a patient-centered care coordination model that deploys health workers integrated into the healthcare team to target disadvantaged patients for a defined episode of care [18–20]. The specific goal of navigation is to reduce delays in care by identifying and addressing patient-identified barriers to care. Early studies documented the benefits of patient navigation along the breast cancer care continuum, including improvements in mammography screening rates, and reductions in diagnostic delays following abnormal screening tests or treatment initiation [20, 21]. Despite the evidence limitations, including studies with small sample sizes, single-site studies, a lack of valid comparison groups, and the use of disparate outcome metrics that preclude comparison, rapid dissemination of the patient navigation model resulted in the American College of Surgeons (ACoS) Commission on Cancer including patient navigation as an accreditation standard [22] and provisions of the Affordable Care Act specifying patient navigation as a means to address barriers to health services [23].
Implementation of this healthcare delivery model into everyday cancer care practice requires evidence that informs best practice. Thus, there is a critical need for generalizable data that provide evidence which is useful in practice. With limited healthcare resources, it is imperative to understand where in the breast cancer care continuum patient navigation has the greatest impact, and on which populations. Using data from a national multicenter clinical trial, we aim to examine the impact of patient navigation on reaching diagnostic resolution at specific time points after an abnormal breast cancer screening test and explore which groups of vulnerable patients have the greatest benefit in an effort to inform how best to deploy patient navigation resources.
Methods
Design overview
We conducted a secondary analysis of data from the National Patient Navigation Research Program (PNRP), the first multicenter clinical trial to examine the benefits of patient navigation among underserved populations with screening abnormalities suspicious for cancer [24]. We conducted a prospective, random-effects meta-analysis based on data from individual patients to determine the effect of navigation on the likelihood of resolution at three specific time points after the screening abnormality was detected, 60, 180 and 365 days.
We chose a prospective meta-analysis design to augment the findings being reported by the individual centers in the PNRP multi-site investigation [17, 24–29]. A meta-analysis contributes to our understanding of the potential benefits of navigation beyond the center-specific findings because it has the distinct advantage of simultaneously and deliberately considering the individual study results in relation to the parent study and makes transparent the differences and similarities in outcomes observed across the participating study centers. A framework that allows for joint attention to similarities and differences in outcomes across study centers can illuminate what is working across varied settings as well as prompt ideas for hypothesis testing in future studies.
Our prospective meta-analysis overcomes some of the limitations of a more traditional retrospective meta-analysis of published studies in that our sample of studies, though small, was not vulnerable to publication bias or search bias. Furthermore, by selecting studies that are part of a larger, multicenter study, we are choosing only studies that are uniform in both the design and implementation of the intervention and in how the outcomes were measured, thereby reducing important sources of potential heterogeneity. We acknowledge that we combined studies of varying research designs, but there are no technical grounds necessitating the removal of non-randomized, controlled designs from a meta-analysis. At the same time, the dissimilarities in patient and institutional characteristics as well as geographic region strengthen the generalizability of our findings [30]. Our prospective design also gave us access to individual, patient-level data which meant that we were not limited by having only summary statistics to perform our analyses. In addition to the meta-analysis, we pooled data from each site in an effort to try to identify the characteristics of the populations that benefited most from the intervention.
Settings and participants
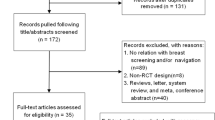
Initially, 10 PNRP centers recruited participants from local community health centers or ambulatory care sites, caring primarily for low-income, uninsured, or publically insured populations [24]. In our analysis, we excluded four centers: one did not enroll breast participants [29], one enrolled too few breast participants to conduct meaningful center-level analyses [28], one did not offer a comparison group amenable to our outcome analysis [31], and one focused on Native American/Alaskan Native communities whose data sharing agreements precluded inclusion in the combined dataset [27]. Eligible participants from the six centers were women 18 years of age or older enrolled with any of the following breast cancer screening abnormality: mammograms, ultrasound, or magnetic resonance imaging (MRI) of the breasts coded by the Breast Imaging Reporting and Data System [32] (BI-RADS) as a 0, 3, 4, or 5 as well as abnormal clinical breast examinations with a mass or other lesion suspicious for cancer (n = 4738). For this analysis, PNRP participants were excluded if they had missing eligibility information (n = 1), if they had a concurrent cervical cancer screening abnormality (n = 14), or because they were no longer able to participate in the study (e.g., deceased, moved out of the country, transferred care to another facility (n = 58). See Fig. 1.
Randomization and interventions
Each of the six centers contributing to this analysis designed study methods in the context of the community setting in which it operated. One center conducted an individually randomized clinical trial [29] requiring written informed consent, two centers conducted a group-randomized trial [20, 28], and three centers used quasi-experimental designs with nonrandom assignment into the intervention and control arms at the group level [17, 27, 31]. The institutional review board for each respective center approved the research.
The patient navigation intervention was modeled after the principles of care coordination [33]. All navigators across the study sites participated in shared face-to-face national trainings held annually and in periodic webinars to ensure standardization across centers [34]. The research team conducted annual core competency assessments on all navigators and provided feedback to local supervisors. Navigation was initiated after a clinician informed the participant of the abnormal test result. Navigators first identified those in need of navigation services, then identified participant-level barriers to recommended care, and developed strategies to address these barriers, with the focus on timely completion of diagnostic evaluation. Navigators were imbedded within clinical care systems with close interface with the clinical practice, and patient follow-up occurred by telephone, mail, and in face-to-face meetings [27, 28, 31, 33–35].
Outcomes and follow-up
All study variable definitions were developed by the PNRP Design and Analysis Committee and implemented uniformly at all centers [24]. Clinical variables were obtained from participants’ medical records, including type of screening abnormality, dates and types of diagnostic test category, and clinical outcomes. Demographics were obtained from either patient self-report or medical record registration.
Dependent variable
The main outcome measure was timely diagnostic resolution of the identified breast abnormality, measured at three specified time points: 60, 180, and 365 days. We chose these endpoints based on their clinical relevance, as reflected in their use as quality metrics by the American Society of Clinical Oncology/National Comprehensive Care Network Quality Measures and the ACoS Commission on Cancer [35], their known effect on survival [4, 36], use for comparison with other studies [37], and practical relevance to implementation. Diagnostic resolution was defined as receipt of a definitive diagnosis (malignant or benign) either through a cytologic or pathologic tissue sample, or through a breast imaging or clinical evaluation that determined no further tests were recommended. For BI-RADS 3 imaging, where short-term imaging is recommended, we tracked participants only through the next recommended imaging. The number of days to resolution was calculated based on the elapsed time between the recorded date of the initial abnormal cancer screening test result and the date of diagnostic resolution. To establish a comparable baseline for BI-RADS 3 cases, where 180 days is the most frequently recommended follow-up period, we subtracted 180 days from the total number of days. For those where resolution was obtained in fewer than 180 days, we assigned them to 0 days to prevent unnatural event times (negative durations). The number of days was used to generate the binary outcome of diagnostic resolution (coded yes/no) for each time period 60, 180, and 365 days.
Independent variable
The main independent variable of interest is whether a patient was in the navigation or control group.
Covariates
A common set of variables collected identically across each study center was included as covariates: age (in years); race/ethnicity (White, African American, Hispanic, or other/unknown); primary language (English or other/unknown); health insurance status (private insurance, public insurance, no insurance); and type of screening abnormality (clinical breast examination, BI-RADS 0/unknown, BI-RADS 3, BI-RADS 4/5).
Statistical analysis
We use descriptive statistics to report socio-demographic characteristics of participants and t tests and Chi-square to compare them by enrollment status (navigated vs. control). For the meta-analysis, we used a two-step approach. First, we analyzed each center independently using a multivariable logistic regression model for each of the specified time points (60, 180, and 365 days). All models were adjusted for a subject’s race/ethnicity, primary language, insurance status, age, and type of cancer screening abnormality. This step produced a mean treatment effect estimate and its standard error for each center. Secondly, we performed a random-effects meta-analysis in order to generate an overall treatment effect for each specified time point (60, 180, and 365 days). We fit a random-effects model to acknowledge that the effects being estimated were not identical across centers [38] since we anticipated heterogeneity among the centers [26], and a random-effects model reduces the weights of individual studies, thus minimizing the likelihood that any obtained significant overall effect is a function of a single center. We further investigated the influence of each study on the overall estimate by omitting one study at a time in a sensitivity analysis. Note that in the rare instances where data about a covariate were missing among the 4675 subjects (i.e., n = 10 for BI-RADS; n = 14 for age; n = 18 for primary language; n = 33 for insurance status; and n = 44 for race/ethnicity), we simply collapsed the missing cases into the “Other” categories since the amount of missing data was negligible.
To explore characteristics of participants who benefited most from the navigation intervention, we then pooled data from all sites. First, we simply compared rates of resolution at each of the three time points for both intervention and control participants. Then, we looked specifically at the subset of participants who had not yet achieved diagnostic resolution by 180 days, as a means to isolate the period demonstrating a benefit of navigation. Data management and statistical calculations were performed with Stata software (version 11.2-SE; Stata Corporation, College Station, TX).
Results
Table 1 displays the demographic characteristics of the 4675 participants and enrollment across all centers. The mean age of women was 48.7 years [standard deviation (SD) 12.1 years], and most were non-White and either uninsured or had publicly funded health coverage. The majority were enrolled based on an abnormal finding on a screening mammogram vs a clinical breast examination finding. Compared with controls, navigated participants were more likely to be younger, Hispanic or other race/ethnicity, be uninsured, and have an abnormal clinical breast examination as their eligibility criteria.
Figures 2, 3, and 4 use forest plots to report the meta-analysis of the adjusted odds ratios (aORs) for timely diagnostic resolution at 60, 180, and 365 days, respectively, where adjusted odds greater than one indicate a benefit in the navigated arm. At 60 days and 180 days of care, there was no statistically significant effect of navigation on achieving timely diagnostic care, but a statistically significant benefit of navigation was seen at 365 days. At 60 days, the aOR for achieving diagnostic resolution was 1.01 [95 % confidence interval (CI) 0.84, 1.22]. At 180 days, the aOR for achieving diagnostic resolution was 1.37 (95 % CI 0.99, 1.88). At 365 days, the aOR for achieving diagnostic resolution was 2.12 (95 % CI 1.36, 3.29), indicating a statistically significant benefit in the navigated arm. There was significant heterogeneity among the centers (I 2 = 69.0 %, p = 0.006).
While our pooled effect size did not show an overall benefit of navigation at 60 days or 180 days, several sites did demonstrate benefit at these specified time frames. For example, site B had an aOR of 1.98 (95 % CI 1.05, 3.73) at 60 days, aOR of 3.17 (95 % CI 1.36, 7.39) at 180 days, and aOR of 8.63 (95 % CI 1.87, 39.80) at 365 days. Similar results are seen for sites C and F. Further, the direction of the association was consistent with an increasing benefit of navigation over time. Influence analyses, which systematically omitted each of the six centers, always produced an effect size within the confidence interval of the original calculation.
To explore factors associated with diagnostic resolution of a breast cancer screening abnormality at 365 days, we display results from each individual study center logistic regression models (Table 2). We did not find race/ethnicity, language, insurance status, or type of screening abnormality to be independently associated with timely diagnostic resolution at 365 days.
We pooled data across all six centers to explore characteristics of the participants who benefited most from the navigation intervention. At 60 days, a little more than half of all enrolled participants achieved diagnostic resolution (58 % in the intervention arm and 56 % in the control arm). At 180 days, almost three quarters of each group achieved diagnostic resolution (76 % intervention arm versus 70 % control arm). At 365 days, 92 % of the navigated participants achieved diagnostic resolution compared with only 84 % of the control participants achieving diagnostic resolution.
Figure 5 displays the results of our pooled data analyses, looking specifically at the participants who benefited from the intervention, which includes the 1252 participants who had not yet achieved diagnostic resolution by 180 days. Figure 4 compares the percent of PNRP intervention and control participants achieving diagnostic resolution between 180 and 365 days across each demographic and clinical group. Overall, only 45 % of those in the control group achieved diagnostic resolution by 365 days, compared with 64 % of intervention participants. With the exception of participants enrolled with a BI-RADS 4/5 screening test (n = 41), each group demonstrated a benefit of navigation. For example, among patients within the control group, 42 % of non-English speakers reached diagnostic resolution after 180 days (by 365 days); by contrast, among patients within navigated group, 66 % of non-English speakers reached diagnostic resolution (Fig. 5).
Discussion
This is the first meta-analysis performed to examine the association between patient navigation and diagnostic resolution at specified time points after an abnormal breast cancer screening test. Among a diverse population of vulnerable women, benefits of the navigation intervention were suggested only among the group that had not yet achieved resolution by 180 days, yet this impact was seen for all demographic groups. Our data suggest that navigation has the greatest impact on cancer care delivery among the group who would otherwise be lost to follow-up after one year. These findings provide practical implications for programs seeking guidance on how to best implement navigation into everyday practice. Should navigation be implemented as a means to specifically address disparities in cancer outcomes, this study suggests that navigation resources be targeted to those with persistent delays in care.
Study methods are notable for several advantages, compared to existing navigation studies which are limited by single-site studies [20, 25, 37, 39–47]. First, we targeted a racial/ethnically diverse population of vulnerable patients seeking care in safety net settings across the country. Furthermore, our prospective meta-analysis utilized individual participant data [48]. Advantages of this approach include overcoming common limitations of traditional meta-analysis in publication bias and within-study selective reporting. It also allowed us to standardize the analyses (same covariates) across studies.
The lack of demonstrated benefit prior to 365 days may be explained by the ability of participants to overcome obstacles to diagnostic care in the absence of a navigator perhaps due to the presence of existing systems within the healthcare setting that increase the likelihood that women will obtain resolution. We found over half of study participants, regardless of navigation status, achieved diagnostic resolution by 60 days. Perhaps these women have barriers to care that usual care is already adept at addressing. There are emerging data to suggest that certain barriers to care, such as housing, disability or employment issues, are more common among those who initially delay care [49]. Since we did not collect information on barriers in control participants, we are unable to account for them in these analyses. Another possible consideration is that the group with initial delays require more complex or multi-step diagnostic services to achieve resolution and thus had more opportunity for navigators to provide necessary support services. The PNRP dataset only included information on the final diagnostic test, without accounting for the multiple other clinical, radiographic, or pathologic tests that might have preceded a final diagnosis. Finally, a possible explanation of the lack of a benefit prior to 365 days is delay in the delivery of navigation services. This is unlikely, however, as our enrollment data show that most women (87 %) were enrolled with documentation of navigation interaction by 60 days.
While our pooled effect size demonstrated no overall benefit of navigation at 60 or 180 days, it is important to recognize that several sites did demonstrate benefit at these specified time points. This suggests the presence of unmeasured center-specific differences, at either the level of the patient or health system. For example, the presence of specific barriers to care for participants at these sites may differ from barriers at sites without demonstrated benefit at those earlier time frames. Or perhaps the baseline delays in care were greater for the sites with demonstrated benefit at earlier time frames. Or on the contrary, sites without demonstrated benefit may have had unmeasured system level resources that provided navigation-like services. Understanding these site-level differences is critical in order to disseminate navigation services in the most efficient manner.
Our study demonstrated an association of navigation and timely care specifically among those whose care was not resolved by 180 days, and the benefit was equal across each demographic group. This is important in relation to disparities in breast cancer, because we know that delays from onset of symptoms to first treatment are associated with shorter survival [4, 50]. Our findings have implications for practices across the country in the process of transforming their care delivery systems to achieve expectations set by the Affordable Care Act for improving quality and efficiency of health care [23], as the current evidence for implementing care coordination best practices is lacking [51]. Our multicenter study findings suggest that care coordination programs targeting timely breast cancer diagnosis should consider specifically targeting patient navigation resources to those with initial delays in care, regardless of socio-demographics. Until evidence from multicenter implementation studies are available, studies such as ours are important to inform the translation of existing evidence into everyday practice.
There are limitations to this study. The heterogeneity we observed across centers in the meta-analysis reflects the reality of community-engaged research, which introduces limitations to conduct pooled analyses but increases generalizability. Given differences in contextual factors among the centers and the nature of the navigation intervention, there are likely individualized implementation differences across centers that were not measured. In the presence of heterogeneity, we fit a random-effects meta-analysis. We also attempted to explore the causes of heterogeneity. One potential cause is the methodological diversity of the centers [31], but the six centers examined here are too few to further divide into subgroups by type of study design and then conduct the meta-analysis. Another potential cause of the heterogeneity is site B/C, which has a wide confidence interval around its large effect size. We are reassured in our sensitivity analysis that when we omit the site showing the largest positive benefit of navigation, site B/C, the combined effect remains positive. We have further confidence in the pooled effects from the forest plot at 365 days, which reveal that all of the estimated effects fall on the same side of the unit line, although in three cases the effect was not statistically significant. Moreover, the odds ratios across all six studies are positive, regardless of study design, and thus suggest a consistency in the effect. For these reasons, we have confidence in our average intervention effect.
In conclusion, this study provides the first multicenter evidence to specify where in the diagnostic care spectrum that patient navigation may have a role in addressing outcome disparities. These data suggest that if we target navigation services to those with persistent delays in breast cancer diagnosis, regardless of socio-demographics, we can improve the quality of care delivery that is necessary to ensure equity in breast cancer survival.
References
Hunt BR, Whitman S, Hurlbert MS (2014) Increasing black: white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol 38:118–123
Bayraktar UD, Chen E, Bayraktar S et al (2011) Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer 117:2364–2370
Brawley OW (2002) Some perspective on black-white cancer statistics. CA Cancer J Clin 52:322–325
McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED (2012) Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 30:4493–4500
Fedewa SA, Ward EM, Stewart AK, Edge SB (2010) Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol 28:4135–4141
Gold HT, Thwin SS, Buist DS et al (2009) Delayed radiotherapy for breast cancer patients in integrated delivery systems. Am J Manag Care 15:785
Maly RC, Umezawa Y, Ratliff CT, Leake B (2006) Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer 106:957–965
Nelson A (2002) Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 94:666
Cancer in the poor: a report to the nation (1989) American Cancer Society, Atlanta
Whitman S, Ansell D, Orsi J, Francois T (2011) The racial disparity in breast cancer mortality. J Community Health 36:588–596
Ahuja N, Chang D, Gearhart SL (2007) Disparities in colon cancer presentation and in-hospital mortality in Maryland: a ten-year review. Ann Surg Oncol 14:411–416
Berry DA, Cronin KA, Plevritis SK et al (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792
Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC (2010) Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ Br Med J 340:c845
Clark CR, Baril N, Kunicki M et al (2009) Addressing social determinants of health to improve access to early breast cancer detection: results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. J Women’s Health 18:677–690
Siminoff LA, Rogers HL, Thomson MD, Dumenci L, Harris-Haywood S (2011) Doctor, what’s wrong with me? Factors that delay the diagnosis of colorectal cancer. Patient Educ Couns 84:352–358
Yang R, Cheung MC, Byrne MM et al (2010) Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer 116:2437–2447
Battaglia TA, Bak SM, Heeren T et al (2012) Boston patient navigation research program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomark Prev 21:1645–1654
Freeman HP (2006) Patient navigation: a community centered approach to reducing cancer mortality. J Cancer Educ 21(1Suppl):S11–4
Fowler T, Steakley C, Garcia AR, Kwok J, Bennett LM (2006) Reducing disparities in the burden of cancer: the role of patient navigators. PLoS Med 3:e193
Wells KJ, Battaglia TA, Dudley DJ et al (2008) Patient navigation: state of the art or is it science? Cancer 113:1999–2010
Paskett ED, Harrop J, Wells KJ (2011) Patient navigation: an update on the state of the science. CA Cancer J Clin 61:237–249
Breast Center Standards Manual (2012) National Accreditation Program for Breast Centers, Chicago
Patient Protection and Affordable Care Act (2010) In: U.S.C, ed
Freund KM, Battaglia TA, Calhoun E et al (2008) National cancer institute patient navigation research program. Cancer 113:3391–3399
Ell K, Vourlekis B, Lee P-J, Xie B (2007) Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med 44:26–33
Freund KM, Battaglia TA, Calhoun E et al (2014) Impact of patient navigation on timely cancer care: the patient navigation research program. J Natl Cancer Inst 106:dju115
Markossian TW, Darnell JS, Calhoun EA (2012) Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomark Prev 21:1691–1700
Paskett ED, Katz ML, Post DM et al (2012) The Ohio patient navigation research program: does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomark Prev 21:1620–1628
Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D (2012) Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomark Prev 21:1629–1638
Walker E, Hernandez AV, Kattan MW (2008) Meta-analysis: its strengths and limitations. Clevel Clin J Med 75:431
Dudley DJ, Drake J, Quinlan J et al (2012) Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer Epidemiol Biomark Prev 21:1639–1644
D’orsi CJ, Radiology ACo, Radiology ACo, Committee B-R (1998) Illustrated breast imaging reporting and data system: (illustrated BI-RADS). American College of Radiology
Longest B, Young G (2000) Coordination and communication. In: Shortell S (ed) Health care management: organizational design and behavior. Delmar Publishers, Albany, pp 237–275
Calhoun EA, Whitley EM, Esparza A et al (2010) A national patient navigator training program. Health Promot Pract 11:205–215
Serfaty M, Wilkinson S, Freeman C, Mannix K, King M (2012) The ToT study: helping with Touch or Talk (ToT): a pilot randomised controlled trial to examine the clinical effectiveness of aromatherapy massage versus cognitive behaviour therapy for emotional distress in patients in cancer/palliative care. Psycho-Oncol 21:563–569
Battaglia TA, Burhansstipanov L, Murrell SS, Dwyer AJ (2011) Assessing the impact of patient navigation. Cancer 117:3551–3562
Psooy BJ, Schreuer D, Borgaonkar J, Caines JS (2004) Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Can Assoc Radiol J 55:145–150
Sedgwick P (2015) Meta-analyses: what is heterogeneity? Br Med J 350:h1435
Battaglia TA, Roloff K, Posner MA, Freund KM (2007) Improving follow-up to abnormal breast cancer screening in an urban population. Cancer 109:359–367
Ferrante JM, Chen P-H, Kim S (2008) The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health 85:114–124
Freeman HP, Muth BJ, Kerner JF (1994) Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice 3:19–30
Ell K, Vourlekis B, Muderspach L et al (2002) Abnormal cervical screen follow-up among low-income Latinas: project SAFe. J Women’s Health Gender Based Med 11:639–651
Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ (2015) Patient navigation to improve follow-up of abnormal mammograms among disadvantaged women. J Women’s Health 24:138–143
Highfield L, Rajan S, Valerio M, Walton G, Fernandez M, Bartholomew L (2015) A non-randomized controlled stepped wedge trial to evaluate the effectiveness of a multi-level mammography intervention in improving appointment adherence in underserved women. Implement Sci 10:143
Drake BF, Tannan S, Anwuri VV et al (2015) A community-based partnership to successfully implement and maintain a breast health navigation program. J Community Health 40:1216–1223
Hunt BR, Allgood KL, Kanoon JM, Benjamins MR (2015) Keys to the successful implementation of community-based outreach and navigation: lessons from a breast health navigation program. J Cancer Educ. doi:10.1007/s13187-015-0904-2
Ramirez A, Perez-Stable E, Penedo F et al (2014) Reducing time-to-treatment in underserved Latinas with breast cancer: the six cities study. Cancer 120:752–760
Riley RD, Lambert PC, Abo-Zaid G (2010) Meta-analysis of individual participant data: rationale, conduct, and reporting. Br Med J 340:c221. doi:10.1136/bmj.c221
Primeau SW, Freund KM, Ramachandran A et al (2014) Social service barriers delay care among women with abnormal cancer screening. J Gen Intern Med 29:169–175
Richards M, Smith P, Ramirez A, Fentiman I, Rubens R (1999) The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer 79:858
Jackson GL, Powers BJ, Chatterjee R et al (2013) The patient-centered medical home: a systematic review. Ann Intern Med 158:169–178
Funding/support
Funding Sources Supported by NIH Grants U01 CA116892, U01 CA117281, U01CA116903, 01CA116937, U01CA116924, U01CA116885, U01CA116875, U01CA116925, American Cancer Society, including #SIRSG-05-253-01, the Avon Foundation and the Boston Medical Center Carter Disparities Fund.
Additional contributions
The authors greatfully acknowledge Richard Campbell for his statistical support and the contributions of the following members of the Patient Navigation Research Program.
Patient Navigation Research Program Investigators:
Clinical Centers Boston Medical Center and Boston University: Karen M Freund (principal investigator (PI)) and Tracy A Battaglia (co-PI); Denver Health and Hospital Authority: Peter Raich (PI) and Elizabeth Whitley (co-PI); George Washington University Cancer Institute: Steven R Patierno (PI), Lisa M Alexander, Paul H Levine, Heather A Young, Heather J Hoffman, and Nancy L LaVerda; H. Lee Moffitt Cancer Center and Research Institute: Richard G Roetzheim (PI), Cathy Meade, and Kristen J Wells; Northwest Portland Area Indian Health Board: Victoria Warren-Mears (PI); Northwestern University Robert H. Lurie Comprehensive Cancer Center: Steven Rosen (PI) and Melissa Simon; Ohio State University: Electra D. Paskett (PI); University of Illinois at Chicago and Access Community Health Center: Elizabeth Calhoun (PI) and Julie Darnell; University of Rochester: Kevin Fiscella (PI) and Samantha Hendren; University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center: Donald Dudley (PI), Kevin Hall, Anand Karnard, and Amelie Ramirez. Program Office National Cancer Institute, Center to Reduce Cancer Health Disparities: Martha Hare, Mollie Howerton, Ken Chu, Emmanuel Taylor, and Mary Ann Van Dyun. Evaluation Contractor NOVA Research Company: Paul Young and Frederick Snyder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
At all sites with with individual randomization, informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Battaglia, T.A., Darnell, J.S., Ko, N. et al. The impact of patient navigation on the delivery of diagnostic breast cancer care in the National Patient Navigation Research Program: a prospective meta-analysis. Breast Cancer Res Treat 158, 523–534 (2016). https://doi.org/10.1007/s10549-016-3887-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3887-8