Abstract
In murine models, overexpression of the MET receptor transgene induces tumors with human basal gene expression characteristics supporting MET inhibition as a treatment strategy for triple-negative breast cancer (TNBC). Foretinib is an oral multi-kinase inhibitor of MET, RON, AXL, TIE-2, and VEGF receptors with anti-tumor activity in advanced HCC and papillary renal cell cancer. Patients with centrally reviewed primary TNBC and 0–1 prior regimens for metastatic disease received daily foretinib 60 mg po in a 2-stage single-arm trial. Primary endpoints were objective response and early progression rates per RECIST 1.1. In stage 2, correlative studies of MET, PTEN, EGFR, and p53 on archival and fresh tumor specimens were performed along with enumeration of CTCs. 45 patients were enrolled with 37 patients having response evaluable and centrally confirmed primary TNBC (cTNBC). There were 2 partial responses (ITT 4.7 % response evaluable cTNBC 5.4 %) with a median duration of 4.4 months (range 3.7–5 m) and 15 patients had stable disease (ITT 33 %, response evaluable cTNBC 40.5 %) with a median duration of 5.4 months (range 2.3–9.7 m). The most common toxicities (all grades/grade 3) were nausea (64/4 %), fatigue (60/4 %), hypertension (58/49 %), and diarrhea (40/7 %). Six serious adverse events were considered possibly related to foretinib and 4 patients went off study due to adverse events. There was no correlation between MET positivity and response nor between response and PTEN, EGFR, p53, or MET expression in CTCs. Although CCTG IND 197 did not meet its primary endpoint, the observation of a clinical benefit rate of 46 % in this cTNBC population suggests that foretinib may have clinical activity as a single, non-cytotoxic agent in TNBC (ClinicalTrials.gov number, NCT01147484).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is defined by <1 % cells with estrogen/progesterone (ER/PR) receptor expression and lack of HER2 overexpression and/or gene amplification and accounts for roughly 15 % of all invasive breast cancers [1, 2]. In the metastatic setting, TNBC has been associated with a shorter duration of response to chemotherapy and poorer overall survival outcomes compared to either ER/PR+ and/or HER2+ subtypes [3]. In the adjuvant setting, systemic risk reduction relies on cytotoxic chemotherapy as there are no effective targeted systemic therapies for TNBC.
MET is a receptor tyrosine kinase preferentially expressed on epithelial and endothelial cells with the sole known ligand being hepatocyte growth factor (HGF). Ligand binding to MET triggers autophosphorylation and dimerization of tyrosine residues in the kinase, leading to downstream phosphorylation of different tyrosine residues in the multi-functional docking site. Subsequent docking of transduction molecules, such as growth factor receptor-bound protein 2 (GRB2) and GRB2-associated protein 1 (GAB1), initiates signaling pathways involved with cellular proliferation, migration, and invasion. MET overexpression has been observed in a number of solid malignancies including non-small cell lung, gastric, pharyngeal, and breast cancer [4–6].
In breast tissue, a spectrum of MET overexpression has been observed with lowest rates in normal breast epithelium, intermediate rates in ductal carcinoma in situ (DCIS), and highest rates in invasive breast cancers. In one of the largest studies to date, MET immunohistochemistry was evaluated in 1274 invasive breast tumors (153 of which were basal-like) and was found to be independently associated with basal-like breast cancer, as well as being an independent adverse prognostic factor across all invasive disease subtypes [7].
Currently, two broad classes of MET inhibitors are in clinical development: monoclonal antibodies and small molecule kinase inhibitors. Foretinib (GSK1363089) is an oral multi-kinase inhibitor primarily targeting MET and vascular endothelial growth factor receptor tyrosine kinase families, but also exerting less potent inhibition of PDGFR, AXL, RON, RET, FLT3, and the receptor of angiopoietin-2 (TIE-2). The main anti-tumor impact of foretinib is due to its activity as a high affinity competitive inhibitor that binds to the ATP pocket of both MET and VEGFR-2, thereby exerting effects on cellular proliferation and invasion, as well as angiogenesis [8].
Six phase I and II studies of foretinib have been completed across a number of tumor types with the recommended continuous daily dose of foretinib being 80 mg of the bisphosphonate salt formulation (GSK1353089A), corresponding to a bioequivalent dose of 60 mg of the foretinib free base formulation (GSK1363089G) [9].
We conducted a multi-centered phase II trial of oral foretinib for locally recurrent or metastatic TNBC.
Patients and methods
IND 197 was a single-arm, non-blinded multicenter phase II trial to investigate the efficacy of continuous single-agent foretinib in patients with incurable TNBC, conducted by the CCTG. A multinomial stopping rule incorporating primary endpoints of both objective response and early progression (at or before 8 weeks after starting treatment) was employed in a 2-stage design [10, 11].The study was conducted according to Good Clinical Practice guidelines with full research ethics board approval at each of the participating institutions. All patients signed written informed consent before study entry.
Eligibility
Patients had to have histologically confirmed locally recurrent or metastatic, incurable TNBC. Central confirmation of TNBC status on the primary tumor was undertaken after patient registration on the trial. In cases where central testing did not confirm TNBC, the treating investigator was informed and patients on treatment could continue if in the opinion of the investigator it was appropriate to do so. Archival tissue samples were collected for translational studies in all patients and those entered on the second stage of accrual had to have an accessible tumor lesion for re-biopsy, and blood collection for circulating tumor cells, prior to study entry. Patients had to have measurable disease by RECIST 1.1 [12] with an ECOG performance status of 0–2 and may have received one prior line of chemotherapy in the metastatic setting. No prior therapy with a MET or angiogenesis inhibitor was permitted. The protocol was amended on December 6, 2010 to exclude patients with a history of deep venous thrombosis or pulmonary embolus diagnosed and/or treated within 6 months prior to registration due to a 9.9 % rate of pulmonary embolism in a single-phase 2 trial of foretinib for papillary renal cell carcinoma. The rate across all other tumor types with foretinib was 3 %, similar to that observed in advanced malignancy.
Study design and treatment plan
Foretinib 60 mg daily was administered in 4 week cycles. Treatment was continued until disease progression, intercurrent toxicities, unacceptable adverse events, decision to withdraw from the study, or inability to continue treatment.
Up to 38 evaluable patients, unselected for MET status, were planned to be accrued with 23 entering the first stage. The study drug would have been considered inactive for the unselected TNBC population if, at the end of the first stage, no responses were identified and ≥17 early progressions occurred. Prior to study closure in that eventuality, MET expression would be assessed in the primary tumors, and if 15 or more were MET negative, a MET-enriched population with up to 23 IHC MET-positive cases were to be enrolled. If ≥1 response or <17 early progressions were observed in stage 1, the study would proceed to stage 2 during which an additional 15 patients were planned to be accrued; 15 unselected TNBC if enrichment for MET was not required in Stage 1 or 15 MET-positive cases if enrichment was required. The study drug would be accepted as active if, in the final sample of 38 patients, there were ≥5 responses or ≤17 early progressions.
Management of toxicity
Up to two dose reductions were permitted for toxicity management as follows: level 1, 45 mg daily and level 2, 30 mg daily. Dose reductions for toxicities were maintained. Grade 2 liver function abnormalities were managed by dose reduction. Other grade 3 toxicities led to a 1 week treatment hold until recovered to < grade 2 with no dose adjustment and a one dose level reduction for a second event. Any grade 4 event led to protocol discontinuation. Patients experiencing grade 1 or greater hypertension were managed according to a specific hypertension algorithm as well as holding foretinib for grade 3 hypertension refractory to medical therapy or grade 4 readings. Blood pressure was monitored daily for those experiencing hypertension until readings were < grade 2. Patients requiring a delay of >2 weeks or >2 dose reductions of foretinib were removed from protocol therapy. Patients experiencing ocular symptoms had foretinib held and a full ophthalmologic assessment was conducted to ascertain etiology and causality with treatment resumption only upon discussion with the CCTG central office.
On study evaluation
Radiologic assessment was performed at baseline, at the end of every second cycle (every 8 weeks), or more often if there was clinical suspicion of progressive disease (PD). Tumor response was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) criteria 1.1 [12]. Stable disease required a minimum of 4 weeks duration. Hematology and biochemistry were evaluated weekly in cycle 1 and on day 1 of subsequent cycles. Thyroid function was evaluated on day 1 of cycle 1 and then on day 1 of every third cycle. Coagulation parameters were evaluated twice weekly for cycle 1 then on day 1 of subsequent cycles for patients taking oral anti-coagulants. Blood pressure and heart rate were assessed weekly for cycle 1 and then every 2 weeks of each subsequent cycle. Ophthalmoscopy was performed on day 1 of every third cycle. Urinalysis was done on day 1 of each cycle. Patient review and toxicity assessment were carried out on day 1 of each cycle and graded according to the National Cancer Institute Common Terminology Criteria Version 3.0. All patients were seen 4 weeks after completion of protocol therapy. Continued follow-up was not required for patients off protocol treatment with PD except to document late toxicities and death. Patients who went off protocol treatment with complete or partial response or stable disease required follow-up every 3 months until PD or death.
Correlative analysis
Archival (all study participants) and fresh tissue (participants enrolled to stage II) was analyzed for MET, epidermal growth factor receptor (EGFR), PTEN and p53 expression by immunohistochemistry (IHC) using monoclonal antibodies against cMET (clone SP44; Ventana, Tucson, AZ), PTEN (clone 138G6; Cell Signaling technology, Beverly MA), EGFR (clone 31G7; Zymed Laboratories, S. San Francisco, CA), and P53 (DO-7, Vector Laboratories, Burlingame, CA). The stainings were conducted using the Ventana BenchMark Autostainer as per the company’s recommended protocol (SP44), or as reported previously (PMID: 22982652 (PTEN), 21352589 (MET), 19884551 (EGFR), 18024870 (P53)). The expression levels of EGFR and MET were assessed semi-quantitatively using the standard H-score (value ranged from 0 to 300). MET positivity was defined as H-score ≥200. EGFR H-score was analyzed as a continuous variable. PTEN was defined as negative (loss) when complete lack of staining in the tumor cells was noted (ref PMID 22982652).
Gene copy changes for EGFR, PTEN, and MET were evaluated using fluorescence in situ hybridization (FISH), as previously described [PMID: 22982652 (PTEN), 19884551 (EGFR)]. MET FISH analysis was performed using the Vysis MET SpectrumRed and CEP7 (D7Z1) SpectrumGreen probe (Abbott Molecular, Des Plaines, IL). EGFR copy number changes were categorized as amplified/high polysomy versus other, using the Colorado University Scoring System [13]. MET was classified as positive if there were on average ≥5 copies/tumor cells (PMID: 19255323).
For a subset of those entered on the second stage, CTCs were isolated from 7.5 mL of blood using microfiltration. Filters were stained using a 3-color immunofluorescence assay staining for cytokeratin (CK), MET, and DAPI, and the entire filter was assessed for the presence/number of CTCs as well as their characteristics. CTCs were enumerated as CTCs based on DAPI-positivity, CK positivity, and/or morphology consistent with a tumor cell (vs a hematopoietic cell). Identified CTCs were then assessed for MET expression. MET positivity was defined as the presence vs absence of staining with intensity not taken into account due to difficulties in assessing this parameter with immunofluorescence vs immunohistochemical techniques.
Fishers exact or the Wilcoxon test was used to examine the association of all correlative analyses with response.
Results
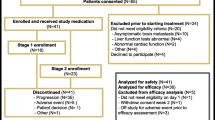
After evaluation of patients enrolled in the first stage, IND197 proceeded to stage 2 in an unselected TNBC population with a total of 45 patients accrued from 9 participating centers across Canada enrolled between September 2, 2010 and August 2, 2013. Two patients did not have TNBC on central review due to minor discrepancies in PR status. All patients receiving at least one dose of foretinib are included in the toxicity analyses. The primary response analysis was performed on the intent to treat population (ITT) which included all 45 registered patients, and a sensitivity analysis was undertaken to evaluate response in the 37 patients with response evaluable and centrally confirmed TNBC (cTNBC).
Patient characteristics
Table 1 summarizes baseline patient and disease characteristics of all subjects. The median age was 55 years (range 29–81 years) and the majority had an ECOG performance status of 0 (62 %). Most tumors were invasive ductal carcinomas (93 %) and grade 3 (76 %). A minority (31 %) had first line chemotherapy for metastatic disease prior to registration.
Toxicity
The majority of the adverse events considered related to foretinib were ≤ grade 2 (Tables 2, 3). The most common grade 3 non-hematological toxicities included hypertension (49 %) and diarrhea (7 %). Two cases of grade 3 nausea, fatigue, dyspnea, and thromboembolism and single cases of grade 3 heart failure, QTc prolongation, anorexia, dehydration, proteinuria, and pleural effusion were observed. An analysis of highest blood pressures revealed six patients with at least one reading of >180 systolic and six with at least one reading of >110 diastolic. There were six serious adverse events considered related to foretinib. Four patients (8.9 %) went off study due to adverse events.
Hematologic toxicity was mild with five patients having asymptomatic grade 3 lymphopenia and two having asymptomatic grade 3 neutropenia. Of those evaluable for toxicity, 53 % received at least 90 % of planned dose intensity and 11 patients had no dose modifications.
Efficacy
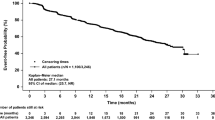
Of the 45 patients enrolled in IND 197, 37 had response evaluable disease and cTNBC. Two partial responses (ITT 4.7 %; response evaluable cTNBC 5.4 %) with a median duration of 4.4 months (range 3.7–5 m) were observed and 15 patients (ITT 33 %; response evaluable cTNBC 40.5 %) experienced stable disease with a median duration of 5.4 months (range 2.3–9.7 m) (Table 3). Overall PR + SD rate was 38 % (95 % CI 24–52 %) in the ITT population and 46 % (95 % CI 30–62 %) in the response evaluable cTNBC population. The Kaplan–Meier estimate of progression-free survival for the ITT population is presented in Fig. 1 (median PFS 1.9 m; 95 % CI 1.8–3.2 m). The maximal decrease in tumor measurements based on investigator assessment, and including only those with repeated assessments of all disease, is presented in the waterfall plot (Fig. 2).
Correlative analysis
Correlative analyses results are summarized in Table 4. Only 3 archival cases were MET IHC positive and all 18 fresh biopsy specimens were negative with 100 % concordance between archival and fresh biopsies. There was no apparent correlation between MET status and response (p = 0.46) and both patients that had a partial response had MET-negative disease. A subset of patients had blood for CTC enumeration collected at baseline. Of these, 13 were negative (<5 CTC) and 4 were positive with no association between baseline CTC count and response. MET expression by IHC was evaluated in all cases with 1 or more CTC and all cases were classed as positive. The discordance between MET IHC expression in biopsy specimens and CTCs is of uncertain significance but may merit further investigation.
There were no significant associations between disease response and PTEN or EGFR IHC or FISH status. Both cases that responded to foretinib had p53-negative disease and no PTEN loss, with a statistically significant association with p53 IHC status but conclusions are limited due to small numbers.
Discussion
TNBC accounts for approximately 15 % of new breast cancer diagnoses. Although clinically conceived as a homogeneous population, there is considerable complexity within the TNBC subset due to incomplete overlap with basal-like gene profiles, an association with BRCA-related hereditary breast–ovarian cancer syndromes and the fact that a small proportion of clinically defined TNBC cases have low-level ER/PR and/or HER2 overexpression and/or gene amplification [14].
The MET signaling pathway is increasingly recognized as being of biologic importance in an array of malignancies, including TNBC, due to MET-dependant cellular processes promoting invasive growth including apoptotic resistance, cellular proliferation, tissue infiltration, and stimulation of angiogenesis [4]. A recent series evaluating 1115 tumor specimens from patients in the MD Anderson Phase 1 Clinic observed that only 5 % of 64 breast cancer cases were MET-amplified [15]. Another series of 1274 invasive breast cancers was assessed for MET expression, correlated with breast cancer subtype and observed independent association of MET expression with basal-like breast cancer (OR = 6.44, 95 % CI 1.74–23.78, p = 0.05), along with higher MET scores being independently associated with worse outcome in the full cohort [7].
Despite increasing understanding of the molecular basis of TNBC, there remains no targeted agent approved for this disease, either as single agent or in combination with chemotherapy. Most clinical trial data in TNBC is derived from subset analyses within a diverse breast cancer patient cohort with trials of cytotoxic chemotherapy alone documenting objective response rates ranging from 10 to 30 % and PFS ranging from 1.6 to 6.2 months [16]. The largest head-to-head trial of single-agent chemotherapy (Carboplatinum-AUC 6 vs Docetaxel 100 mg/m2) in metastatic TNBC reported to date randomized 376 patients and observed no significant differences in ORR (36 vs. 31 %) or PFS (4.8 vs. 5.2 m) in the overall trial population [17].
A systematic review of phase 3 randomized controlled trials of biological agents in addition to chemotherapy versus chemotherapy alone in metastatic TNBC has recently been published. The authors observed a significant improvement in PFS (HR 0.65; 95 % CI 25–43 %) with the addition of bevacizumab to chemotherapy with no impact on OS. There were no significant benefits observed with the addition of sunitinib, sorafenib, lapatinib, iniparib, or cetuximab to chemotherapy on either PFS or OS [18]. Importantly, IND 197 stands alone as a trial of a single-agent, non-cytotoxic-targeted therapy in this disease subset.
Both archival and fresh biopsy specimens were collected from the relevant patient populations on either Stage 1 or Stage 2 of IND197. Similarly to the MD Anderson cohort which documented MET amplification via FISH in 3 of 64 patients with breast cancer (5 %), we observed a low rate of MET IHC-positive tumors (6.7 %) with no association between MET status and best response. Of note, cross-study comparisons are limited due to significant variability in MET assessment methodologies and scoring systems. We also did not observe any association between non-progression (PR + SD) and EGFR, p53, PTEN status in either the primary or freshly biopsied metastatic tumors, or with MET-positive CTC number, among those entered on to Stage 2 of the trial.
Although CCTG IND 197 did not meet its primary endpoint, the observation of a clinical benefit rate of 46 % in the response evaluable cTNBC population, in the context of this particular breast cancer subset with suboptimal outcomes, suggests a degree of clinical activity unique for a non-cytotoxic single agent. This is supported by data in the waterfall plot (Fig. 2) although anti-tumor activity estimated by waterfall plots may appear to be superior to RECIST criteria as only the best change in sum of diameters, without need for confirmation, is plotted. Grade 3 hypertension (HTN) was observed in 49 % of patients, consistent with VEGF targeting of foretinib, but responded to medical management and was not associated with HTN-related complications nor did it lead to study withdrawal in any cases. Unfortunately, we were unable to confirm a predictive biomarker for clinical benefit and suggest, as do others, that putative predictive biomarkers be evaluated in large pre-clinical panels with standardized methodologies in attempts to optimize potential clinical benefit [19]. Our study suggests that further research assessing foretinib versus placebo subsequent to first line chemotherapy for metastatic TNBC in efforts to improve progression-free survival may be warranted.
References
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26:2568–2581
Metzger-Filho O, Tutt, de Azambuja E et al (2012) Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol 30:1879–1887
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Appleman LJ (2011) MET signaling pathway: a rational target for cancer therapy. J Clin Oncol 29:4837–4838
Maroun CR, Rowlands T (2014) The MET receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther 142:316–338
Blumenschein GR, Mills GB, Gonzalez-Angulo AM (2012) Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol 30:3287–3296
Ho-Yen CM, Green AR, Rakha EA et al (2013) C-met in invasive breast cancer. Cancer 120:163–171
Qian F, Engst S, Yamaguchi K et al (2009) Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res 69:8009–8016
Zhu K, Kong X, Zhao D et al (2014) C-MET kinase inhibitors: a patent review (2011–2013). Expert Opin Ther Pat 24:217–230
Dent S, Zee B, Dancey J et al (2001) Application of a new multinomial phase II stopping rule using response and early progression. J Clin Oncol 19:785–791
Freidlin B, Dancey J, Korn EL (2002) Multinomial phase II designs. J Clin Oncol 20:599
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Capuzzo F, Hirsch FR, Rossi E et al (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. H Natl Cancer Inst 97(9):643–655
Mayer IA, Abramson VG, Lehmann BD et al (2014) New strategies for triple negative breast cancer-deciphering the heterogeneity. Clin Cancer Res 20:782–790
Jardim DL, Tang C, Gagliato DDM et al (2014) Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson phase I clinic. Clin Cancer Res 20:6336–6345
Gelmon K, Dent R, Mackey JR et al (2012) Targeting triple-negative breast cancer: optimizing therapeutic outcomes. Ann Oncol 23:2223–2234
Tutt A, Ellis P, Kilburn L et al (2014) TNT: a randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA ½ breast cancer (CRUK/07/012). 2014 San Antonio Breast Cancer Symposium, San Antonio TX, USA. Publication number S3-01, Dec 9–13
Bramati A, Girelli S, Torri V et al (2014) Efficacy of biological agents in metastatic triple-negative breast cancer. Cancer Treat Rev 40:605–613
Gaule PB, Crown J, O’Donovan N et al (2014) CMET in triple-negative breast cancer: is it a therapeutic target for this subset of breast cancer patients? Expert Opin Ther Targets 18:999–1009
Acknowledgements
Canadian Cancer Trials Group was supported by the Canadian Cancer Society Research Institute to the Canadian Cancer Trials Group (grant #021039).
Funding
PB was funded by a Cancer Care Ontario Research Chair in Experimental Therapeutics. Fellow (PB) was funded by AstraZeneca—CCTG Drug Development Fellowship and the Terry Fox Foundation Training Program in Transdisciplinary Cancer Research in partnership with CIHR, Canadian Cancer Trials Group Development Fellowship
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Potential conflict of interest statements
Dr. Rayson has received honoraria from Ipsen, Amgen, and Novartis and has served as a consultant for Ipsen and Novartis. Dr Lupichuk has served as a consultant for Genomic Health. Dr Potvin has received honoraria from Novartis, Pfizer, and Janssen and has been on a speakers’ bureau for Novartis and Pfizer. Dr Dent has received honoraria from Roche, Amgen, and Astra Zeneca and has served as a consultant for Novartis. Dr Shenkier has received honoraria from Novartis and Roche and has served as a consultant for Novartis, Roche, and Janssen. Dr Ellard owns stock in Pfizer, Abbvie, and GSK and has served as a consultant for GSK. Dr Prady has served as a consultant for AstraZeneca. Dr Allo reports spousal employment with GSK. Dr Goodwin has served as a consultant for Celgene, Novartis, BMS, Amgen, and Ipsen. Drs Dhesy-Thind, Salim, Farmer, Tsao, Allan, Ludkovski, Bonomi, Tu, Eisenhauer, and Bradbury report no potential conflicts of interest as does Ms. Hagerman. GSK provided foretinib and supplied funding to the Canadian Cancer Trials Group to support IND 197 but was not involved with the trial design, data analysis, or reporting of results.
Rights and permissions
About this article
Cite this article
Rayson, D., Lupichuk, S., Potvin, K. et al. Canadian Cancer Trials Group IND197: a phase II study of foretinib in patients with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2-negative recurrent or metastatic breast cancer. Breast Cancer Res Treat 157, 109–116 (2016). https://doi.org/10.1007/s10549-016-3812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3812-1