Abstract
Breast cancer in young women has been shown to have an aggressive behavior and worse prognosis. Studies evaluating young women enrolled in clinical trials of neoadjuvant chemotherapy have shown that age is a determinant factor in the achievement of a pathological complete response (pCR). In this study, we sought to analyze the outcomes of young patients treated with neoadjuvant chemotherapy at a single institution. 1639 patients treated with neoadjuvant chemotherapy were included. 316 patients ≤40 years were compared with 1323 patients aged >40 years regarding the achievement of a pCR (defined as no invasive residual tumor in the breast or lymph nodes). Disease-free survival (DFS) and overall survival were compared between groups according to pCR status and subtype, defined by hormone receptor (HR) and HER2 status. Young women were more likely to have a pCR than their older counterparts (37.4 vs. 26.3 %, P < 0.001). This difference was significant both for HR+/HER2− and triple-negative (TN) tumors. Young age and achieving less than pCR were associated with a greater chance of recurrence for the entire population. Age was not an independent factor for recurrence in TN and HER2+ disease. However, being younger than 40 increased recurrence risk in HR+/HER2− tumors. The achievement of a pCR was not associated with improved DFS in young women with HR+/HER2− tumors. Although young women have a high rate of pCR, they also have a worse prognosis. In a real-world clinical setting, the achievement of a pCR was an independently significant protective factor for recurrence across all subtypes and ages, except for HR+, HER2− disease in young women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer in young women has been shown to have a worse prognosis and is related with a higher incidence of hormone receptor negative tumors, a higher histologic grade, advanced disease presentations, and a worse disease-free survival [1–4]. This predominance of aggressive subtypes is illustrated by the fact that young women have a higher risk of recurrence and death than older women [5]. Although guidelines suggest that biology should be the main driver of treatment among young patients and not age itself, the best treatment for this population is still controversial, particularly regarding the efficacy of cytotoxic treatment in different breast cancer subtypes.
Neoadjuvant chemotherapy has become the standard of care for the treatment of patients with locally advanced breast cancer and for specific breast cancer subtypes, in which the achievement of a pathologic complete response (pCR) has been found to correlate with a better prognosis [6, 7]. pCR is dependent on several factors, including hormone receptor (HR) status, human epidermal receptor type-2 (HER2) expression and the type of treatment used [8]. Recently, it has been recognized that age is another determinant factor for the achievement of a pCR, as younger women are more likely to achieve a pCR with neoadjuvant chemotherapy [9]. However, there are insufficient data to suggest that the achievement of a pCR after neoadjuvant treatment is a marker of better outcome for young women.
In this study, we sought to compare the outcomes of young patients with different breast cancer subtypes treated with neoadjuvant chemotherapy, and to compare them with their older counterparts.
Methods
We conducted an observational retrospective cohort study including all patients with a diagnosis of breast cancer treated with neoadjuvant chemotherapy at the Instituto Nacional de Cancerología (INCan), a comprehensive cancer center in Mexico City, between January 2007 and December 2013. Approval for this study was obtained from the Ethics Committee at INCan. Patient characteristics including stage at diagnosis, type of treatment, histopathological analysis (including immunophenotype), site of relapse, disease-free survival (DFS), and overall survival (OS) were recorded. Patients were divided between two groups according to age: ≤40 years old and >40 years old. Those ≤40 were considered to be young breast cancer patients.
Dedicated breast pathologists performed all histologic assessments. Estrogen receptor (ER) and progesterone receptor (PR) were determined by immunohistochemistry (IHC). [10], and receptor status was assessed using the Allred Score [11]. An IHC score of greater than 2 (corresponding to as few as 1–10 % positive cells) was used to defined ER and Pr positivity. Cases with an Allred score of 2 or lower were classified as HR negative. For HR-positive cases, we used the semiquantitative scoring method or “H-score” to define the intensity of receptor expression. This method provides an overall score (0–300) based on the sum of ordinal weighted percentiles of cells stained weak, moderate, and strong [12]. Since Ki67 staining was not used for all cases, we used the H-Score to classify HR-positive tumors into the Luminal A and Luminal B subtypes according to previously published methodology [12]. Those tumors with an H-score above 200 were considered Luminal A, while those with an H-score under 200 were considered Luminal B. HER2 was determined initially by IHC and considered negative in cases of 0 (no membrane staining) or 1+ (weak and incomplete membrane staining) scoring. Tumors were considered HER2+ in cases of 3+ IHC staining or amplified FISH and HER2− in cases with 0, 1+ and 2+ IHC plus negative FISH amplification [13]. Cases with negative ER, PR, and HER2 were considered to be triple-negative breast cancer (TNBC).
Clinical and radiographic staging procedures were used for all patients. T stage was defined using either clinical or ultrasound measurements. Clinical N stage was defined by either the presence of palpable axillary lymph nodes or of abnormal lymph nodes upon ultrasound examination. Metastatic disease was evaluated using imaging as indicated.
Pathological complete response (pCR) was defined as no invasive residual tumor in the breast or lymph nodes [noninvasive breast residuals allowed (ypT0/is, ypN0)]. The presence of invasive residuals in either the breast or axilla was considered as residual invasive disease. After completion of treatment patients were followed up, using current international recommendations. Hormonal therapy was prescribed to all patients with HR+ disease. DFS was calculated from the time of surgery to the date of first identification of recurrent disease or last relapse-free visit, and OS was calculated from the date of diagnosis to the date of last visit or death.
All data are presented as medians, means, or proportions. Descriptive statistics, multivariate analysis, and regressions were carried out using R version 3.1.3 (R Project, www.r-project.org). Ggplot2 software (R Project, www.ggplot2.org) was used for plotting graphs. The Chi-square test was used to compare the distribution of baseline characteristics among groups according to age (≤40 years vs. >40 years). Kaplan–Meier analysis was used to calculate survival outcomes. In order to estimate relapse and survival risk between different subgroups, we used a Cox proportional hazards regression model. All P values presented are 2 sided, and P values < 0.05 were considered statistically significant.
Results
During the studied period, 1639 women with breast cancer were treated with systemic neoadjuvant chemotherapy at INCAN. Median age at diagnosis was 48.9 years (range 42.2–56.7 years). 1323 patients (80.7 %) were older than 40 years of age and 316 (19.3 %) were 40 years of age or younger. Median follow-up was 50.8 months (range 27.1–70.4 months).
The most common histologic subtype was HR+, HER2− (55.5 %, n = 910), followed by HER2+ (26.2 %, n = 430) and TNBC (17.5 %, n = 287). While HR+, HER2− tumors were the most commonly found subtype in both groups, the proportion of this subtype was significantly smaller in younger women (57.3 % in >40 years vs. 48.1 % in ≤ 40 years, P < 0.001), while for TNBC, the proportion was higher in younger patients (15.7 % in >40 years vs. 25 % in ≤40 years, P < 0.001). Younger patients were more likely to have more aggressive tumors, with a larger proportion of T3 tumors and high grade neoplasms. The characteristics of the patients are presented in Table 1. Among young women with HR+, HER2− tumors (n = 152), 29.6 % (n = 45) were classified as Luminal A and 70.4 % (n = 107) as Luminal B according to H-Score, in comparison with 41.2 and 58.8 % respectively in women over 40 (P = 0.01, Table 2).
In the young women group, 118 patients (37.4 %) achieved a pCR, compared with 348 patients (26.3 %) in the >40 group (P < 0.001). Only 10 patients overall had a ypTis response after neoadjuvant chemotherapy, of which 4 were in the ≤40 group and 6 in the >40 group. pCR was associated with age (Fig. 1), with an OR of 1.5 (95 % CI 1.1–2.0) in younger women. This difference was largely driven by higher rates of pCR both in the HR+, HER2− (23.3 vs. 13.6 %, P = 0.02) and in the TN subtypes (56.4 vs. 41 %, P = 0.02). Differences in pCR according to subtype in both groups are shown in Table 3. When the HR+, HER2− subtype was analyzed, we found that while both Luminal A and Luminal B tumors had a higher possibility of obtaining a pCR in younger women, this difference was only statistically significant for the Luminal A subtype, although the number of such tumors was small (Table 3).
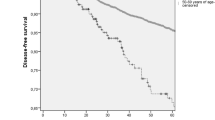
The achievement of a pCR was generally associated with better DFS and OS regardless of age. Although young women achieved higher pCR rates, DFS was shorter for young women when compared to their older counterparts (5 year DFS 69.2 vs. 77.6 %, P = 0.006) (Fig. 2). Both young age and achieving less than a pCR were independently associated with a greater chance of recurrence when taking into account all tumor subtypes. When the influence of both age and residual disease on DFS for each particular subtype was analyzed using multivariate analysis (Table 4), we found that being younger than 40 years increased the risk of recurrence only for the HR+, HER2− subtype, but not for the HER2+ or TN subtypes (Fig. 2). The achievement of a pCR remained as an independently significant protective factor for recurrence across all subtypes and all ages, except for HR+, HER2− disease in young women (Fig. 3). In this group, 5-year DFS was 63 % (95 % CI 41.2–96.5) for those with a pCR and 64.5 % (95 % CI 53.9–77.1) for those with less than pCR (P = 0.34) (Fig. 3). In contrast to DFS, age was not a predictor of worse OS (5 year OS 83.6 vs. 86.3 %, P = 0.5) when all tumor subtypes were analyzed, and this remained true for each individual subtype (Fig. 4; Table 5). The only factor associated with OS in our analysis was the achievement of a pCR, with a 5-year OS of 95 % (95 % CI 92.7–97.6) for those achieving a pCR versus 82.3 % (95 % CI 79.6–85.1) for those without a pCR (Fig. 5).
Discussion
In this study, pCR rates were significantly higher in young women than in their older counterparts. This difference in pCR was significant for TN and HR+, HER2− tumors, while there was no difference in HER2+ disease. Despite having higher pCR rates than older women, young women with breast cancer also showed higher rates of recurrence than their older counterparts. Interestingly, this difference in DFS was mainly driven by those patients with HR+, HER2− tumor, while age appears to have no effect on the recurrence rate of HER2+ and TNBC.
The earliest report on the outcomes of young women undergoing neoadjuvant chemotherapy for breast cancer was published in 1999. In this study, despite a good control of the primary tumor by chemotherapy, young women had a significantly higher risk of relapse than older women [14]. Of note, the patients included in this analysis were not given adjuvant hormonal therapy. The importance of adjuvant endocrine treatment added to chemotherapy in young women with ER+ tumors has been emphasized by results from the International Breast Cancer Study Group, which found that chemotherapy alone was not as effective as chemotherapy and endocrine therapy for young women with breast cancer [15].
Recently, the German Breast Group reported the pooled analysis of the outcomes of young women treated with neoadjuvant chemotherapy in the context of several neoadjuvant clinical trials [9]. Similar to our results, they found a significantly higher rate of pCR in women under 40 when compared with those 40–49 years of age and those 50 and older (20.9 vs. 17.7 vs. 13.7 %; P < 0.001), which was confined to TNBC and HR+/HER2− tumors. Notably, the definition of pCR used by the authors was ypT0, ypN0, which is stricter than the one used in our research and in other publications [16]. One of the key findings in this analysis was that young women with HR+/HER2− tumors who failed to achieve a pCR had a worse DFS than those without residual disease (in contrast to the previously reported data by the same group pointing toward the fact that pCR in this subtype is not correlated with DFS [8]). Although these results were unexpected, the authors attributed their results to a different biology of breast cancer in young women, which could be caused by a higher prevalence of Luminal B type tumors (in which pCR has been associated with better survival outcomes [8]) among younger patients [1, 17, 18].
Contrary to those findings, in our analysis, we failed to see an improvement in 5-year DFS when patients with HR+, HER2− disease, and pCR were compared to those with residual disease. This difference could be attributed to the use of different definitions of pCR, although due to the low number of patients with a ypTis response in our study, this seems unlikely. The most plausible explanation is the fact that patients in the German report were treated within the context of various clinical trials in which patients with HR-positive disease received adjuvant hormonal therapy in a controlled environment. In contrast, while our patients were prescribed adjuvant hormonal therapy according to current guidelines, the possibility of poor compliance is greater in a real-world clinical setting. Although the magnitude of benefits of 5 years of adjuvant tamoxifen appears to be similar in younger compared with older women [19], the difference in DFS found between age groups in HR+, HER2− tumors could be explained both by a lower adherence to treatment in younger women [20] and/or by the use of the more effective aromatase inhibitors in older women [21]. Hopefully, these differences in outcomes may change in the future with the use of the combination of an aromatase inhibitor and ovarian suppression as adjuvant treatment for premenopausal women with breast cancer [22].
Our analysis failed to show a difference in OS between younger and older women, regardless of tumor subtype; the only factor that predicted better OS was the achievement of a pCR. This might be explained by the fact that most of the difference in DFS rates was driven by HR+, HER2− tumors, which are characterized by having late relapses, good responses to treatment in the metastatic setting and long median survivals [23]. Thus, the fact that we found no difference in OS may be due to our relatively short follow-up time.
Limitations of our study included its retrospective design, the lack of central pathology review of all cases, the lack of a more precise characterization of each subtype, and a relatively short median follow-up. Additionally, there was no information regarding the full range of treatment, such as radiotherapy dosing for each individual patient or data regarding adherence to hormonal treatment.
This analysis has several strengths. In contrast with the recently published results in which patients were highly selected [9], our data come from a heterogeneous patient population. Despite the fact that our patients received a variety of treatments and were not under the strict supervision of a clinical trial, our results are consistent to those previously reported [9]. This is important because it provides real-world data pointing the fact that there are indeed important unresolved issues in the management of HR+, HER2− breast cancer in young women, and that this group of patients should be studied in depth in future trials of neoadjuvant therapies.
In conclusion, our results confirm that although young women have a high rate of pCR (which is confined to TN and HR+, HER2− tumors), they also have higher recurrence rates. Young women with HR+, HER2− breast cancer treated with neoadjuvant chemotherapy have a higher risk of relapse than their older counterparts in a real-world clinical setting, in contrast to those with HER2+ and TNBC. This is relevant because it identifies a gap in treatment that should be closed by testing new therapies in this population of patients in order to improve their outcomes. Furthermore, our results show that additional data are needed to determine if the achievement of a pCR after neoadjuvant treatment is a marker of better outcomes for young women with HR+, HER2− breast cancer, and whether the lack of a pCR should be used or not to select those younger patients in need of a more aggressive approach in the adjuvant setting.
References
van der Hage JA, Mieog JS, van de Velde CJ, Putter H, Bartelink H, van de Vijver MJ (2011) Impact of established prognostic factors and molecular subtype in very young breast cancer patients: pooled analysis of four EORTC randomized controlled trials. Breast Cancer Res 13:R68
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG et al (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330
Azim HA, Partridge AH (2014) Biology of breast cancer in young women. Breast Cancer Res 16:427
Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP et al (2012) Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 131:1061–1066
Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M (2000) Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ 320:474–478
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, Colleoni M, Denkert C, Eiermann W, Jackesz R et al (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18:1927–1934
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B et al (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26:814–819
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kümmel S, Schneeweiss A, Huober J, Hilfrich J, Hanusch C et al (2015) Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 152:377–387
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134:e48–e72
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Cohen DA, Dabbs DJ, Cooper KL, Amin M, Jones TE, Jones MW, Chivukula M, Trucco GA, Bhargava R (2012) Interobserver agreement among pathologists for semiquantitative hormone receptor scoring in breast carcinoma. Am J Clin Pathol 138:796–802
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P et al (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138:241–256
Braud AC, Asselain B, Scholl S, De La Rochefordière A, Palangie T, Dieras V, Pierga JY, Dorval T, Jouve M, Beuzeboc P et al (1999) Neoadjuvant chemotherapy in young breast cancer patients: correlation between response and relapse? Eur J Cancer 35:392–397
Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thürlimann B, Rudenstam CM, Lindtner J, Crivellari D, Cortes-Funes H et al (2000) Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 355:1869–1874
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Park YH, Lee SJ, Jung HA, Kim SM, Kim MJ, Kil WH, Lee JE, Nam SJ, Ahn JS, Im YH (2015) Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: single institutional experience in Korea. Breast 24:213–217
Tang LC, Jin X, Yang HY, He M, Chang H, Shao ZM, Di GH (2015) Luminal B subtype: a key factor for the worse prognosis of young breast cancer patients in China. BMC Cancer 15(1):201
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784
Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol 23:882–890
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M et al (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509–518
Pagani O, Regan MM, Francis PA et al (2014) Exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371:1358–1359
Migliaccio I, Malorni L, Hart CD, Guarducci C, Di Leo A (2015) Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med 13:46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical standards
All of the procedures included in this report comply with current Mexican laws. Approval for this study was obtained from the Ethics Committee at INCan.
Rights and permissions
About this article
Cite this article
Villarreal-Garza, C., Bargallo-Rocha, J.E., Soto-Perez-de-Celis, E. et al. Real-world outcomes in young women with breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 157, 385–394 (2016). https://doi.org/10.1007/s10549-016-3811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3811-2