Abstract
Studies on active and passive tobacco smoking and breast cancer have found inconsistent results. A meta-analysis of observational studies on tobacco smoking and breast cancer occurrence was conducted based on systematic searches for studies with retrospective (case–control) and prospective (cohort) designs. Eligible studies were identified, and relative risk measurements were extracted for active and passive tobacco exposures. Random-effects meta-analyses were used to compute summary relative risks (SRR). Heterogeneity of results between studies was evaluated using the (I 2) statistics. For ever active smoking, in 27 prospective studies, the SRR for breast cancer was 1.10 (95 % CI [1.09–1.12]) with no heterogeneity (I 2 = 0 %). In 44 retrospective studies, the SRR was 1.08 (95 % CI [1.02–1.14]) with high heterogeneity (I 2 = 59 %). SRRs for current active smoking were 1.13 (95 % CI [1.09–1.17]) in 27 prospective studies and 1.08 (95 % CI [0.97–1.20]) in 22 retrospective studies. The results were stable across different subgroup analyses, notably pre/post-menopause, alcohol consumption adjustments, including/excluding passive smokers from the referent group. For ever passive smoking, in 11 prospective studies, the SRR for breast cancer was 1.07 (95 % CI [1.02–1.13]) with no heterogeneity (I 2 = 1 %). In 20 retrospective studies, the SRR was 1.30 (95 % CI [1.10–1.54]) with high heterogeneity (I 2 = 74 %). Too few prospective studies were available for meaningful subgroup analyses. There is consistent evidence for a moderate increase in the risk of breast cancer in women who smoke tobacco. The evidence for a moderate increase in risk with passive smoking is more substantial than a few years ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women in the world, representing one quarter of all cancers diagnosed in women in 2012 [15]. Among the lifestyle risk factors for breast cancer, Danaei et al. [11] found that 21 % of breast cancer deaths are attributable to alcohol consumption, overweight and obesity and lack of physical activity. Observational studies on active or passive smoking and breast cancer, as well as meta-analyses and reviews have reached a wide range of conclusions, from an unlikely association to suggestion of a causal association [9, 21, 25, 32, 36, 43]. The Surgeon General’s Report [42] is the most recent large review on the topic that reviewed the literature until 2012, finding a small but significant increased risk of breast cancer for active smokers and suggesting a possible association between passive smoking and breast cancer in pre-menopausal women.
The aim of this study was to systematically review and perform meta-analyses on the relationship between active and passive tobacco exposures and breast cancer occurrence, based on an exhaustive search of observational studies published up to March 2015.
Methods
Studies published up to March 2015 were identified through a search of PUBMED and Web of Science databases with the keywords: “smoking”, “tobacco smoke pollution”, “tobacco use”, “tobacco products” and “breast neoplasms”, using both MeSH terms for the PUBMED search and other possible synonyms (e.g. “breast carcinoma”). Additional records were manually identified searching the references of published articles, reviews and previous meta-analyses.
Study eligibility criteria
Literature searches focused on prospective (cohorts, nested case controls, case-cohorts) and retrospective (case–controls) observational studies. Ecological and cross-sectional studies were excluded. Case control studies were excluded if (i) not enough information was available regarding cases and controls selection; (ii) the control selection was likely to be biased (e.g. low response rate, controls unrepresentative of the cases); (iii) the breast cancer cases were prevalent and not incident. Breast cancer cases were considered incident if the time between breast cancer diagnosis and the interview to ascertain tobacco exposure data was at most 6 months. Active smoking exposure had to be reported as ever, former, or current smoking. Passive smoking exposure was extracted as having ever been exposed to second-hand smoke during lifetime or the most comprehensive indicator of passive smoking exposure.
Studies had to report an estimate for the relative risk of incident invasive breast cancer for those exposed to tobacco (actively or passively) compared to those never exposed (actively or passively, respectively) or sufficient data to compute a risk estimate. Studies on mortality, in situ carcinoma, or on patients with previously existing breast cancer were excluded.
Data extraction
Data on study and population characteristics, outcomes, exposures, risk estimates and confidence intervals were extracted, with verification by a second reviewer.
When risk parameters were reported by subgroup, the relative risk and 95 % CI for the entire study were computed using a fixed effects meta-analysis. If risk parameters were reported according to the same referent category, the method proposed by Greenland et al. [17] for computing a relative risk and 95 % CI for the entire study was used. When risk parameters were not reported, the article was searched for data allowing the calculation of unadjusted odds ratios (OR) and relative risks.
Statistical analyses
No distinction was made between various risk parameters (odds ratio, risk ratio, rate ratio, relative risk). The risk parameters and their corresponding variances were log transformed. Summary relative risks (SRR) were computed using random-effects meta-analysis models [44]. The between-study variance was computed using a restricted maximum likelihood estimator [45], and confidence intervals were calculated using the weighted variance method [19, 40].
Heterogeneity of effects across studies was evaluated using the I 2 statistic, which represents the proportion of total variation in the estimates of effects that is due to heterogeneity between study results rather than to chance [20]. Subgroup meta-analyses were done to explore possible sources of heterogeneity. Publication bias was investigated using three tests [3, 14, 30] and visual assessment of funnel plots. Sensitivity analyses were carried out to assess the impact of each individual study on the summary risk estimate through the “leave-one-out” method.
To investigate the evolution of the knowledge on the association between active or passive smoking and breast cancer, cumulative temporal meta-analyses were conducted by including studies progressively by year of publication. In this procedure, the summary relative risk and its confidence interval are re-calculated each time that a more recent study is included in the group of studies subjected to the meta-analysis.
A dose–response meta-analysis was carried out for the association between the duration of ever active smoking and breast cancer risk using the method described by Greenland et al. [17]. This method requires that data be available for the number of cases, person-years, relative risk estimates and their variances for at least three quantitative exposure categories. For articles lacking information on either the person-years or the number of cases per exposure category, but reporting the total number of cases and person-years, the missing data were estimated [2]. Durations of smoking reported in categories were transformed in continuous variables calculated as the average of the upper and lower bounds of the categories. When the highest category was open ended, the duration assigned to that category was estimated as the value of the upper bound multiplied by 1.2. For the dose–response meta-analysis, a linear model was used to estimate the increment in relative risk associated with each additional year of ever active smoking.
All statistical analyses were done using the R 3.1.3 software.
Results
The literature search returned 1639 articles, and 51 additional articles were identified through references search (Fig. 1). The systematic screening of titles and of abstracts led to the exclusion of 1416 articles. 274 articles were reviewed full text. One hundred eighty-eight articles did not meet eligibility criteria. A final set of 86 articles related to independent studies were selected for inclusion in the meta-analysis. Seventy-five studies (31 prospective, 44 case–controls) investigated the association between active smoking and breast cancer risk, and 31 studies (11 prospective and 20 retrospective) investigated the association between exposure to passive smoking and breast cancer incidence (some studies reported data on both types of exposure). The full list of articles with their main characteristics and tobacco smoking data that were reported are summarized in the Supplementary material, Table S1.
Four separate meta-analyses were done comparing ever, or former, or current active smoking exposure vs. never active smokers and exposure of never smokers to second-hand smoking vs. absence of exposure to second-hand smoking. The summary relative risks (SRR) and their confidence intervals are summarized in Table 1. Forest plots for ever active or passive smoking in all studies are displayed in Figs. 2 and 3.
For active smoking, there were more breast cancer cases in prospective than in retrospective studies (Table 1). Results of meta-analyses were indicative of statistically significant moderate increased risks of breast cancer associated with ever, current and former active tobacco smoking. The highest SRR of 1.13 (95 % CI: 1.09–1.17) was found for current active smoking in prospective studies. The heterogeneity of results between studies was more manifest for retrospective designs. For all but one of these analyses, all three publication bias tests indicated no evidence of publication bias for a cut-off p value of 0.1. For former active smoking among prospective studies, the Egger test suggested the presence of publication bias; however, the Begg and Macaskill tests did not. The leave-one sensitivity analysis did not identify any one study that strongly influenced the results.
In the temporal cumulative meta-analysis (Figure S1), it is apparent that the association between ever smoking and breast cancer was already present and statistically significant in 1992, with a SRR of 1.10. This SRR remained at around 1.10 since then, with a progressive narrowing of the confidence interval due to the accumulation of breast cancer cases. In prospective studies, the increased summary relative risk is manifest since the first six studies were published in the early 2000’s (Figure S2).
Because heterogeneity of results was high in retrospective studies, stratified analyses were performed for prospective studies only (Table 2). A summary relative risk estimate (SRR) for ever active smoking of about 1.10 was consistently retrieved in studies done in North America and in Europe, in pre- or post-menopausal women, and in studies that adjusted or did not adjust for alcohol consumption. A meta-analysis of five studies that reported risk estimates using never active or passive smokers as the referent group obtained a SRR of 1.13 (95 % CI [1.04; 1.22]). In contrast, when never active, but ever passive smokers was used as the referent group, a similar SRR of 1.10 (95 % CI [1.09; 1.12]) was obtained. Only three studies examined the smoking breast cancer association in women who never drink alcohol. The small number of studies and the overall number of cases they included (5947) precluded a meaningful analysis in this subgroup.
Results for subgroup analyses for current and former active smoking were similar to those for ever active smoking, with the SRRs being slightly greater for current active smoking (data not shown).
Results of meta-analyses were indicative of statistically significant moderate increased risks of breast cancer associated with exposure to second-hand tobacco smoke (Table 1). The heterogeneity of results between studies was confined to retrospective designs. The presence of publication bias was unlikely. The leave-one sensitivity analysis did not identify any one study that strongly influenced the results.
In the temporal cumulative meta-analysis (Figure S3), the association between passive smoking and breast cancer emerged in 2009, after which the SRR continued to increase while the confidence interval narrowed. In prospective studies, it is only when the most recent study published in 2014 was included in the meta-analysis that the SRR became statistically significant (Figure S4).
Because heterogeneity of results was high in retrospective studies, stratified analyses for prospective studies only were performed (Table 3), but these analyses were hampered by the small number of studies that reported stratified relative risks.
Data of 12 prospective studies allowed to examine the relationship between the duration of ever active smoking and the risk of breast cancer (Figs. 4 and 5). Assuming a linear dose–response relationship, the slope parameter β provides a quantitative estimate of the incremental increase in the risk of breast cancer associated with each additional year of active smoking.
Forest plot of prospective studies investigating the association between the duration of ever actively smoking and breast cancer risk. The linear trend slope estimate (β) and their 95 % CI from each study are shown by a square and segments, respectively. The overall summary linear trend slope estimate is represented by a rhombus (Summary β). Summary β represents the incremental increase in breast cancer risk per year of ever active smoking
Relative risk of breast cancer incidence in function of the duration of ever actively smoking (in years) among 12 studies with prospective designs. The colour of the points indicates the original study, and the size of the points is inversely proportional to the variance of the RR estimate given in this study. The linear regression trend line is drawn in black, using the summary slope estimate β = 1.005, and the dotted lines represent the 95 % confidence interval of the slope estimate
The summary slope estimate was 1.005 (95 % CI [1.003; 1.007]), indicating that with every additional year of active smoking the relative risk of breast cancer incidence is multiplied by 1.005 (Fig. 4). As a consequence, the risk of breast cancer in women who smoke during 10, 20 or 30 years would be increased by 5, 10 and 16 %, respectively.
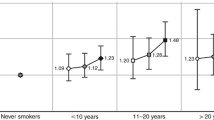
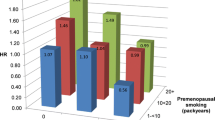
A plot of breast cancer risk against smoking duration shows that the dose–response relationship was quite consistent in larger studies [4, 8, 10, 29, 35, 47]. In contrast, the relationship was usually erratic in smaller studies [1, 7, 16, 27, 31, 38], which contributed to most of the heterogeneity of 65 % between results of individual studies (Fig. 4). Similar results were obtained (based on 11 studies) when pack-years of smoking of ever actively smoking were used as the exposure measure (see Supplementary material Figures S5 and S6).
Nine prospective studies reported the relative risk of breast cancer in function of age at smoking initiation. In order to compare the relative risks for smoking initiation before and after 20 years old, two separate random meta-analyses were computed, one for each category. The summary relative risks were both indicative of a positive significant association between ever active smoking (with initiation before or after 20 years old) and breast cancer, with a point estimate greater for smoking initiation before 20 years old (SRR = 1.11 (95 % CI [1.07; 1.15]) vs SRR = 1.07 (95 % CI [1.05; 1.10])) (see Supplementary material Figures S7 and S8). Although for this analysis ever smokers (with initiation before or after 20 years old) were not separated into former or current smokers, this finding would be consistent with an increased risk of breast cancer with longer smoking duration.
Discussion
The results of the present meta-analysis provide evidence that active cigarette smoking would be associated with a moderate, statistically significant increased risk of breast cancer. Both retrospective and prospective observational designs led to the same conclusion. The summary relative risk estimates have remained remarkably stable over time, and the accumulation of studies has steadily reduced the variance of estimated risks. The likelihood of an association is further supported by the quasi-absence of heterogeneity in results of prospective studies and by the stability of results across different subgroups, notably in pre-menopausal and post-menopausal women, after adjustment for alcohol consumption and when passive smokers were excluded from the referent group.
Exposure to environmental tobacco smoke also seems associated with a moderately increased risk of breast cancer. However, results for passive smoking are more delicate to interpret because of the difficulty to assess exposure to second-hand smoking exposures and of the relatively small number of available studies. Summary relative risk estimates have increased over time, and it is only after 2008 that a statistically significant elevated risk has emerged.
One strength of the present meta-analysis is the inclusion of several recently published observational studies, updating the previously published reviews, and the inclusion of older but eligible studies. For both active and passive smoking, summary relative risks estimates have remained quite stable over time, and the accumulation of studies has steadily reduced the variance of estimated risks.
Observational epidemiologic studies have their limitations. Case–control studies collect the information retrospectively, have no follow-up and can be prone to information and selection bias. Prospective studies are less likely to suffer from these biases; however, the exposure is often measured only at the baseline, with no information on the changes in exposure that arise over the course of the follow-up period, leading to possible exposure misclassification. For active smokers, residual confounding is still possible even after adjusting for alcohol consumption. The subgroup analysis among never drinkers lacked sufficient power, being based on only three studies, and further studies in never drinkers are recommended.
Some subgroup analyses were unfeasible because of the limited number of available data. For instance, only few data were found for Asia, none were found for Central and South America and no data were found for low income countries. Only three prospective studies gave results stratified by ER/PR tumour subtype [12, 29, 34]. The small number of prospective studies on passive smoking and breast cancer precluded subgroup analyses having sufficient statistical power.
Active smoking
Alcohol is a known risk factor for breast cancer [13, 18], and it has been shown to be positively correlated with smoking [33]. The Collaborative Group on hormonal factors [18] analysed the association between alcohol, tobacco and breast cancer risk, using individual data from 53 observational studies. They restricted their analysis on the effect of tobacco to only never drinking women and concluded that smoking was not associated with breast cancer for never drinking women. The Collaborative Group [18] also considered all women regardless of alcohol consumption, and the relative risk of breast cancer and active smoking was estimated at 1.09 (95 % CI [1.05; 1.13]) unadjusted for alcohol and 1.05 (95 % CI [1.01; 1.09]) adjusting for alcohol, which is consistent with the results of the current study.
In 2010, the International Agency for Research on Cancer (IARC) published a monograph on carcinogenic risks [22] and found a positive association between tobacco smoking and breast cancer, based on reviewed literature up to 2008. They reported that most cohort studies found an association between longer duration of smoking and greater risk of breast cancer, and that no statistically significant differences were observed in function of age at smoking initiation. The evidence at the time was considered as inconclusive regarding the association with menopausal status. The evidence included in the present meta-analysis was consistent with the previous findings regarding smoking duration and age at initiation and subgroup meta-analyses indicated that ever active smokers are associated with an increased risk of breast cancer, regardless of menopausal status.
The 2014 Surgeon General’s Report [42] investigated the association between smoking and breast cancer risk based on cohort studies published before 2012 and case–control studies published from 2000 to 2011. They concluded that ever active smoking increases the relative risk of breast cancer by a “statistically significant average of 9 %”. Six studies included in the Surgeon General’s Report were excluded from the present analysis on the basis of prevalent cases [6, 26, 37, 39, 41, 48]. The present meta-analysis also includes 13 more recent studies, published between 2011 and 2015, and 35 older studies published between 1984 and 2010. The findings of the present updated meta-analysis, based on a larger sample of studies, reinforce the conclusions of the Surgeon General’s Report [42]: (i) moderate, but statistically significant average increase of the relative risk of breast cancer incidence for active ever/former/current smokers and (ii) dose–response relationship between active smoking intensity and duration, and breast cancer risk. The Surgeon General’s Report [42] concluded that the association between smoking, menopausal status and breast cancer was uncertain. The present study found that the menopausal status does not modify the association between active smoking and breast cancer.
It has been argued that the presence of passive smokers in the referent category could obscure or diminish the association between active smoking and breast cancer risk, especially if passive smoking is also associated with the risk of developing breast cancer [23, 24, 46]. The results of this meta-analysis were not affected by considering only prospective studies that excluded passive smokers from the referent group, showing a moderate, but statistically significant, risk of breast cancer in both cases (passive smokers included or excluded from the referent group), with the heterogeneity among results remaining low.
Passive smoking
In 2010, The IARC monograph [22] reviewed the literature on breast cancer and environmental tobacco smoke and found that the evidence was inconsistent. However, the IARC did not perform meta-analyses. The 2014 Surgeon General’s Report [42] performed meta-analyses of articles published before 2012. The report concluded that studies on the association between passive smoking and breast cancer risk had obtained inconsistent results, with a small increased risk on average that is highly sensitive to study design. Compared to the Surgeon General’s report, five studies were excluded because they were considered to include prevalent cases [26, 28, 39, 41, 48] and five older studies were included that were not considered by the Surgeon General’s report. In addition, data of one cohort study have been updated [12], seven new retrospective studies and one cohort study have been published from 2011 to 2015. Although the number of prospective studies remains too small to conduct meaningful subgroup analyses, the overall summary estimate of the relative risk of breast cancer indicated a moderate, statistically significant increase for passive smokers, with very little heterogeneity among the prospective cohorts’ results.
Biological plausibility
A considerable literature documents the deleterious and carcinogenic effects of tobacco smoking [5, 22]. Concentrations of toxic chemicals are several times higher in side-stream smoke (the smoke produced by an idling cigarette) compared to mainstream smoke (the smoke directly inhaled through the cigarette by an active smoker) [9]. Exposure to environmental tobacco smoke as well as active cigarette smoking seem therefore biologically plausible etiological factors for breast cancer incidence.
Conclusions
As time passes, the evidence accumulates for considering that active tobacco smoking is associated with a modest, but real increase in the risk of breast cancer. The consistency of findings, the low heterogeneity of results of prospective studies, the dose–response found in prospective studies, the permanent higher risk since the first studies done on the topic, and the absence of influence of major confounders on associations are all indicating that the relationship would be causal. For passive smoking also, the evidence for a modest but real association with breast cancer strengthens with the accumulation of data. In this respect, public health policies should inform women about the risk of breast cancer associated with both active and passive smoking.
References
Al-Delaimy WK, Cho E, Chen WY, Colditz G, Willet WC (2004) A prospective study of smoking and risk of breast cancer in young adult women. Cancer Epidemiol Biomarkers Prev 13:398–404
Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T (2012) Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose–response meta-analysis of prospective studies. Ann Oncol 23:843–852. doi:10.1093/annonc/mdr398
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Bjerkaas E, Parajuli R, Weiderpass E, Engeland A, Maskarinec G, Selmer R, Gram IT (2013) Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302,865 Norwegian women. Cancer Causes Control 24:1347–1356. doi:10.1007/s10552-013-0213-1
Boyle P, Gray N, Henningfield J, Seffrin J, Zatoński W (2010) Tobacco: science, policy and public health, 2nd edn. Oxford University Press, Oxford
Brown LM, Gridley G, Wu AH, Falk RT, Hauptmann M, Kolonel LN, West DW, Nomura AM, Pike MC, Hoover RN, Ziegler RG (2010) Low level alcohol intake, cigarette smoking and risk of breast cancer in Asian–American women. Breast Cancer Res Treat 120:203–210. doi:10.1007/s10549-009-0464-4
Catsburg C, Kirsh VA, Soskolne CL, Kreiger N, Rohan TE (2014) Active cigarette smoking and the risk of breast cancer: a cohort study. Cancer Epidemiol 38:376–381. doi:10.1016/j.canep.2014.05.007
Catsburg C, Miller AB, Rohan TE (2015) Active cigarette smoking and risk of breast cancer. Int J Cancer 136:2204–2209. doi:10.1002/ijc.29266
Collishaw NE, Boyd NF, Cantor KP, Hammond SK, Johnson KC, MIllar J, Miller AB, MIller M, Palmer JR, Salmon AG, Turcotte F (2009) Canadian expert panel on tobacco smoke and breast cancer risk., OTRU special report seriesOntario Tobacco Research Unit, Toronto
Cox DG, Dostal L, Hunter DJ, Le Marchand L, Hoover R, Ziegler RG, Thun MJ, Breast Prostate Cancer, Cohort C (2011) N-acetyltransferase 2 polymorphisms, tobacco smoking, and breast cancer risk in the breast and prostate cancer cohort consortium. Am J Epidemiol 174:1316–1322. doi:10.1093/aje/kwr257
Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment collaborating (2005) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366:1784–1793. doi:10.1016/S0140-6736(05)67725-2
Dossus L, Boutron-Ruault MC, Kaaks R, Gram IT, Vilier A, Fervers B, Manjer J, Tjonneland A, Olsen A, Overvad K, Chang-Claude J, Boeing H, Steffen A, Trichopoulou A, Lagiou P, Sarantopoulou M, Palli D, Berrino F, Tumino R, Vineis P, Mattiello A, Bueno-de-Mesquita HB, van Duijnhoven FJ, Bakker MF, Peeters PH, Weiderpass E, Bjerkaas E, Braaten T, Menendez V, Agudo A, Sanchez MJ, Amiano P, Tormo MJ, Barricarte A, Butt S, Khaw KT, Wareham N, Key TJ, Travis RC, Rinaldi S, McCormack V, Romieu I, Cox DG, Norat T, Riboli E, Clavel-Chapelon F (2014) Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int J Cancer 134:1871–1888. doi:10.1002/ijc.28508
Dumitrescu RG, Shields PG (2005) The etiology of alcohol-induced breast cancer. Alcohol 35:213–225. doi:10.1016/j.alcohol.2005.04.005
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Ferlay J, Soerjomataram I, Ervik M, Dikshit RP, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2012) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 In: International Agency for Research on Cancer, Lyon, France
Gram IT, Braaten T, Terry PD, Sasco AJ, Adami HO, Lund E, Weiderpass E (2005) Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev 14:61–66
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, Koetsawang S, Rachawat D, Morabia A, Schuman L, Stewart W, Szklo M, Bain C, Schofield F, Siskind V, Band P, Coldman AJ, Gallagher RP, Hislop TG, Yang P, Kolonel LM, Nomura AM, Hu J, Johnson KC, Mao Y, De Sanjose S, Lee N, Marchbanks P, Ory HW, Peterson HB, Wilson HG, Wingo PA, Ebeling K, Kunde D, Nishan P, Hopper JL, Colditz G, Gajalanski V, Martin N, Pardthaisong T, Silpisornkosol S, Theetranont C, Boosiri B, Chutivongse S, Jimakorn P, Virutamasen P, Wongsrichanalai C, Ewertz M, Adami HO, Bergkvist L, Magnusson C, Persson I, Chang-Claude J, Paul C, Skegg DC, Spears GF, Boyle P, Evstifeeva T, Daling JR, Hutchinson WB, Malone K, Noonan EA, Stanford JL, Thomas DB, Weiss NS, White E, Andrieu N, Bremond A, Clavel F, Gairard B, Lansac J, Piana L, Renaud R, Izquierdo A, Viladiu P, Cuevas HR, Ontiveros P, Palet A, Salazar SB, Aristizabel N, Cuadros A, Tryggvadottir L, Tulinius H, Bachelot A, Le MG, Peto J, Franceschi S, Lubin F, Modan B, Ron E, Wax Y, Friedman GD, Hiatt RA, Levi F, Bishop T, Kosmelj K et al (2002) Alcohol, tobacco and breast cancer–collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer 87:1234–1245. doi:10.1038/sj.bjc.6600596
Hartung J, Knapp G (2001) A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 20:3875–3889
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. doi:10.1002/sim.1186
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 83., Tobacco smoke and involuntary smokingWorld Health Organization, International Agency for Research on Cancer, Lyon
International Agency for Research on Cancer (2009) A review of human carcinogens. Part E: Personal habits and indoor combustions. Lyon, France
Johnson KC (2005) Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer 117:619–628. doi:10.1002/ijc.21150
Johnson KC, Wells AJ (2002) Active and passive smoking in breast cancer. Epidemiology 13:745–746. doi:10.1097/01.ede.0000032423.06738.c4
Khuder SA, Simon VJ Jr (2000) Is there an association between passive smoking and breast cancer? Eur J Epidemiol 16:1117–1121
Kropp S (2002) Active and passive smoking and risk of breast cancer by age 50 Years among German Women. Am J Epidemiol 156:616–626. doi:10.1093/aje/kwf093
Land SR, Liu Q, Wickerham DL, Costantino JP, Ganz PA (2014) Cigarette smoking, physical activity, and alcohol consumption as predictors of cancer incidence among women at high risk of breast cancer in the NSABP P-1 trial. Cancer Epidemiol Biomarkers Prev 23:823–832. doi:10.1158/1055-9965.EPI-13-1105-T
Liu L, Wu K, Lin X, Yin W, Zheng X, Tang X, Mu L, Hu Z, Wang J (2000) Passive smoking and other factors at different periods of life and breast cancer risk in chinese women who have never smoked—a case–control study in Chongqing, People’s Republic of China. Asian Pac J Cancer Prev 1:131–137
Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, Tindle HA, Tong E, Rohan TE (2011) Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ 342:d1016. doi:10.1136/bmj.d1016
Macaskill P, Walter SD, Irwig L (2001) A comparison of methods to detect publication bias in meta-analysis. Statist Med 20:641–654
Manjer J, Johansson R, Lenner P (2004) Smoking is associated with postmenopausal breast cancer in women with high levels of estrogens. Int J Cancer 112:324–328. doi:10.1002/ijc.20409
Miller MD, Marty MA, Broadwin R, Johnson KC, Salmon AG, Winder B, Steinmaus C, California Environmental Protection A (2007) The association between exposure to environmental tobacco smoke and breast cancer: a review by the California Environmental Protection Agency. Prev Med 44:93–106. doi:10.1016/j.ypmed.2006.08.015
Moore AA, Gould R, Reuben DB, Greendale GA, Carter MK, Zhou K, Karlamangla A (2005) Longitudinal patterns and predictors of alcohol consumption in the United States. Am J Public Health 95:458–465. doi:10.2105/AJPH.2003.019471
Nyante SJ, Gierach GL, Dallal CM, Freedman ND, Park Y, Danforth KN, Hollenbeck AR, Brinton LA (2014) Cigarette smoking and postmenopausal breast cancer risk in a prospective cohort. Br J Cancer 110:2339–2347. doi:10.1038/bjc.2014.132
Olson JE, Vachon CM, Vierkant RA, Sweeney C, Limburg PJ, Cerhan JR, Sellers TA (2005) Prepregnancy exposure to cigarette smoking and subsequent risk of postmenopausal breast cancer. Mayo Clin Proc 80:1423–1428. doi:10.4065/80.11.1423
Palmer JR, Rosenberg L (1993) Cigarette smoking and the risk of breast cancer. Epidemiol Rev 15:145–156
Prescott J, Ma H, Bernstein L, Ursin G (2007) Cigarette smoking is not associated with breast cancer risk in young women. Cancer Epidemiol Biomarkers Prev 16:620–622. doi:10.1158/1055-9965.EPI-06-0873
Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, Horn-Ross PL, Peel D, Pinder R, Ross RK, West D, Wright WE, Ziogas A (2004) active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. JNCI J Natl Cancer Inst 96:29–37. doi:10.1093/jnci/djh002
Roddam AW, Pirie K, Pike MC, Chilvers C, Crossley B, Hermon C, McPherson K, Peto J, Vessey M, Beral V (2007) Active and passive smoking and the risk of breast cancer in women aged 36–45 years: a population based case–control study in the UK. Br J Cancer 97:434–439. doi:10.1038/sj.bjc.6603859
Sanchez-Meca J, Marin-Martinez F (2008) Confidence intervals for the overall effect size in random-effects meta-analysis. Psychol Methods 13:31–48. doi:10.1037/1082-989X.13.1.31
Slattery ML, Curtin K, Giuliano AR, Sweeney C, Baumgartner R, Edwards S, Wolff RK, Baumgartner KB, Byers T (2008) Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat 109:101–111. doi:10.1007/s10549-007-9629-1
Surgeon General (2014) Cancer. In: The health consequences of smoking—50 years of progress
Terry PD, Rohan TE (2002) Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev 11:953–971
van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21:589–624
Viechtbauer W (2005) Bias and efficiency of meta analytic variance estimators in the random effects model. J Educ Behav Stat 30:261–296
Wells AJ (1991) Breast cancer, cigarette smoking, and passive smoking. Am J Epidemiol 133:208–210
Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB (2011) Cigarette smoking and the incidence of breast cancer. Arch Intern Med 171:125–133. doi:10.1001/archinternmed.2010.503
Young E, Leatherdale S, Sloan M, Kreiger N, Barisic A (2009) Age of smoking initiation and risk of breast cancer in a sample of Ontario women. Tob Induc Dis 5:4. doi:10.1186/1617-9625-5-4
Acknowledgments
The authors thank Magali Boniol for the data extraction verifications.
Author contribution
A.M. and P.B. designed the study. A.M. did the literature search, extracted and analysed the data. All authors interpreted the results. A.M. wrote the report. All authors reviewed, revised and edited the report.
Funding
This work was founded by the International Prevention Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Macacu, A., Autier, P., Boniol, M. et al. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 154, 213–224 (2015). https://doi.org/10.1007/s10549-015-3628-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3628-4