Abstract
Previous observational studies have suggested that metformin in diabetes patients may reduce breast cancer risk more than the reductions from other anti-diabetes medications. This randomized, double-blind, placebo-controlled trial was performed to evaluate the efficacy of metformin for controlling physical and metabolic profiles related to prognosis and adverse events in non-diabetic breast cancer patients. Female breast cancer patients (N = 105), at least 6 months post-mastectomy, with obesity (≥25 kg/m2) and/or pre-diabetes (fasting blood sugar levels ≥100 mg/dL), were randomly assigned to three groups (placebo, metformin 500 mg, and metformin 1000 mg) stratified by tamoxifen use. A linear mixed model for repeated measurements among three groups and ANOVA for profile differences during 6 months of treatment were used for the intention-to-treat analysis. The metformin 1000 mg group had a significantly greater decline in glucose and HbA1c levels between treatment weeks 0 and 6 month (p = 0.008 and 0.009, respectively), and the declines increased with an increase in body mass index (BMI) level (p interaction with BMI = 0.007 and 0.067, respectively). A marginally significant different effect from the metformin 1000 mg treatment was detected for glucose and HbA1c levels (p interaction = 0.084 and 0.063, respectively) in the intention-to-treat analysis. Metformin 1000 mg treatment had a favorable effect on controlling glucose and HbA1C levels in obese non-diabetic breast cancer patients, indicating prognostic importance. Further trials are needed to elucidate the risk–benefit ratio of long-term use of metformin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metformin (a biguanide derivative) is the most commonly prescribed oral medication used as a first-line treatment choice for type 2 diabetes patients with normal kidney function. Biologically, metformin inhibits the mammalian target of rapamycin (mTOR) signaling pathway, which is the major target for anticancer effects via activation of the adenosine monophosphate-activated protein kinase (AMPK) pathway, a cellular energy sensor [1]. AMPK has a role for peripheral and central energy regulation. In hypothalamus, AMPK inhibited by metformin reduces the appetite, and leptin leads to reduction of body weight. In contrast, metformin activates the AMPK in liver and skeletal muscle. Activated AMPK decreases the fatty acid synthesis, cholesterol synthesis, and gluconeogenesis in liver, and increases the glucose uptake in muscle [2]. The AMPK–mTOR-related mechanism involved in metformin’s effects is similar to the effects of physical exercise (a lifestyle modification) with its glucose-lowering capacity and associated health benefit [3, 4].
There is evidence emerging from experimental studies that metformin can play a crucial anticancer role in breast cancer [5, 6]. Prior observational studies have suggested that metformin treatment in diabetic patients, compared to the effects of other diabetes drugs, can reduce the absolute risk of cancer development [7–10].
Several recent studies have reported the beneficial effects of metformin in breast cancer prognoses, including decreasing the mortality rate in breast cancer patients [7, 9, 10], and on breast cancer-related prognostic factors, such as reducing fasting insulin levels (by 22.4 %) and body weight (by 2.5 %), and in an observational study, improving insulin sensitivity (by 25.6 %) in women with non-metastatic breast cancer [11]. Moreover, it has been reported that diabetic breast cancer patients receiving metformin and neoadjuvant chemotherapy had a higher pathological complete response (24 %) than that in other antidiabetic drug-treated (8 %) or pre-diabetic (16 %) breast cancer patients in a 17-year follow-up dataset that included 2529 breast cancer patients (metformin vs. non-metformin, p = 0.007) [12]. The risk of all-cause mortality was twice as high in breast cancer women with HbA1C ≥7.0 % compared with women with HbA1C less than 6.5 % [13]. Therefore, it is assumed that metformin preferentially modifies physical and metabolic conditions in breast cancer patients, leading to reduce further the recurrence of and mortality from breast cancer.
In this study, we evaluated the efficacy of adjuvant metformin on improving physical conditions in operable, obese, non-diabetic breast cancer patients. The study was not extended to the endpoint of breast cancer patients (i.e., cancer recurrence or overall survival time); instead, our clinical trial investigation focused on the preferential efficacy of metformin on weight loss, improving obesity indices, and on diabetes profiles.
Methods
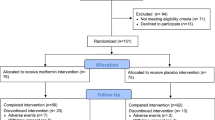
This study is a randomized, double-blind, placebo-controlled trial of operable breast cancer patients. Initially, we evaluated the eligibility of 456 operable breast cancer patients with stage 0 to stage IIIA breast cancer presenting between September 2009 and March 2011 at the Seoul National University Hospital, Seoul, Korea, and who had undergone mastectomy at Seoul National University Hospital at least 6 months prior to evaluation. Of them, 351 patients were ineligible or did not agree to participate in the study. Finally, 105 patients agreed to participate in this trial. In accordance with the guidelines of the Declaration of Helsinki and the National Health and Medical Research Council, all participants provided written informed consent. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (H-0810-007-259), and the study was registered in the www.ClinicalTrials.gov (Identifier: NCT00909506).
Our eligibility criteria were as follows. For inclusion, trial patients were to be obese (body mass index [BMI] >25 kg/m2 [14]) or pre-diabetic (100 mg/dL ≤ fasting blood sugar levels [FBS] <126 mg/dL) women who had undergone breast cancer surgery (mastectomy); aged over 20 years; more than 6 months since breast cancer surgery and at least 4 weeks since prior chemotherapy and/or radiotherapy; without any current medication except tamoxifen; with normal kidney (within a normal range of serum creatinine level) and liver (based on the old tuberculin/prothrombin time test) functions; with Eastern Cooperative Oncology Group performance status of 0–2 or a Karnofsky performance status of 60–100 %; with a life expectancy of over 12 weeks; with an absolute neutrophil count ≥1.5 × 109/L; with a platelet count ≥100 × 109/L; preventing pregnancy during the study period; and willing to sign a written informed consent.
Excluded patients were those diagnosed with diabetes (Types I or II) and/or taking any insulin-related medication; with allergic reactions attributed to chemical or biologic compositions similar to metformin; with any medical history of liver, kidney, cardiac, or infectious diseases or with angina pectoris or lactic acidosis; with prior or current use of metformin or any steroid; pregnant or lactating; with an inability to swallow or digest oral medications; on a diet and/or medication for weight loss; with anorexia and/or adephagia; or using concurrent investigational agents.
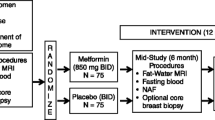
The 105 included female breast cancer patients were randomly allocated to one of three intervention groups stratified by tamoxifen therapy (use vs. non-use) to form a placebo group (N = 35), a metformin 500 mg group (N = 35), and a metformin 1000 mg group (N = 35). In addition, there were three BMI/FBS groups: (1) BMI > 23 and 100 mg/dL ≤ FBS < 126 mg/dL; (2) BMI >23 and FBS <100 mg/dL; and (3) BMI ≤23 and 100 mg/dL ≤ FBS < 126 mg/dL. The stratified randomization was conducted independently by the Medical Research Collaborating Center at Seoul National University Hospital using a web-based randomization protocol. Study medications (metformin and placebo) were provided as identical-appearing capsules in coded containers and were as supplied by the manufacturer (Daewoong Pharmaceutical, Seoul, Korea). Patients, investigators, and collaborators remained blinded throughout the entire trial.
Ninety-six patients (91.4 %) completed at least 12 weeks of treatment (97.1 % in the placebo group; 94.3 % in the metformin 500 mg group; and 82.9 % in the metformin 1000 mg group), whereas 81 patients (77.1 %) continued to take pills for 6 months (80.0 % in the placebo group; 77.1 % in the metformin 500 mg group; and 74.3 % in the metformin 1000 mg group). Intergroup differences in participation rates were not significant (p > 0.1). Self-induced drug stoppage due to no beneficial effect was the most frequent reason for patients not completing the trial in the placebo group (4 of 7 patients), while withdrawal due to adverse events was the most frequent reason in both metformin groups (5 of 8 patients in the metformin 500 mg group and 5 of 9 patients in the metformin 1000 mg group).
Pharmacological intervention
During the first 2 weeks of the trial, participants assigned to the metformin 500 mg and metformin 1000 mg groups identically took 500 mg of metformin after dinner, while participants in the placebo group took a placebo after dinner. After that 2-week period, a second dose of 500 metformin or placebo was added after each breakfast in all groups for a further 22 weeks (Fig. 1).
After random allocation to groups, we followed study participants until the end of trial (6 months) by using three methods. The first follow-up method was direct visits to a coordinating nurse (at baseline (week 0) and at 4 weeks, 12 weeks, and 6 months). The second method was a telephone follow-up every 2 weeks, and the third method was a participants’ self-administrated daily record of the number of taken pills, responses, and adverse events. Individual counseling was administered at each visit to breast cancer clinic by a well-trained research nurse.
Drug adherence was evaluated by counting pills at each patient visit and by telephone monitoring. Study participant adherence was recorded when more than 80 % of the drug dosages were taken during the period following initial contact. In cases exhibiting non-adherence (i.e., low compliance), the participant was counseled on the importance of taking the prescribed amount of the medication. Adverse event occurrences were checked at each visit and during each telephone survey.
Measurement
Serum creatinine levels were measured at baseline to evaluate subjects’ kidney function. The FBS, glycated hemoglobin (HbA1c), and insulin levels were measured at baseline (week 0), week 12, and end of trial (6 months), whereas a lipid profile including total cholesterol, HDL- and LDL-cholesterols, and triglyceride levels was obtained twice (weeks 0 and 6 months). Anthropometry (i.e., weight, height, waist circumference, and blood pressures) and body composition via bioelectrical impedance analysis (Zeus 9.9; Jawon Medical, Kyungsan, Korea) were measured during every visit. The beck depression inventory (BDI) and other demographic factors were collected via questionnaire at baseline and end of trial (Fig. 1).
Sample size and statistical analyses
The required sample size calculation was based on the results of a randomized controlled trial of lifestyle intervention and metformin treatment in schizophrenia patients [15]. In that study, the weight loss after a 12-week trial of metformin 750 mg and placebo treatments averaged −3.2 kg (95 % CI −3.9 to −2.5 kg) and 1.2 kg (95 % CI 2.4–3.8 kg), respectively (Fig. 2). We assumed the metformin effect was on linear decreasing trend from a 0 kg weight loss; therefore, the weight loss values expected in our trial were −2.1 kg for the 500 mg metformin treatment and −3.2 kg for the 1000 mg metformin treatment. Using the analysis of variance (ANOVA) equation for estimating sample sizes suitable for comparisons of three means at the 0.05 level of significance (two-tailed test 0.025) with 90 % statistical power and equal sample number in each group, a sample size of 25 patients per group was calculated. Assuming a 30 % dropout rate, the required sample size per group was 35 patients.
Changes in the repeated physical and metabolic profiles among the three groups throughout the trial were compared by using a linear mixed model, which is a type of repeated measures ANOVA (RMANOVA). To test the difference between the two profiles at baseline and the end of trial among the three treatment groups (24 weeks), we conducted ANOVA (analysis of variance). The distributions in all covariates among the three groups, such as age, tamoxifen use (yes vs. no), smoking status (ever vs. never), regular exercise (yes vs. no), and menopause status (pre- vs. post-), which act as potential risk factors, were not statistically significant; therefore, we did not adjust for these variables. Interactions for changes in the metabolic profiles between baseline and 24 weeks by metformin intervention were evaluated according to hormone receptor, BMI, and TMX use.
All intention-to-treat analyses were performed by using SAS software version 9.2 (SAS Institute, Cary, North Carolina, USA).
Results
All demographic, anthropometric, and clinic-pathological factors were well matched among the three treatment groups (p > 0.05). Stage 0 and I breast cancers were relatively higher in the placebo group (45.7 %) than in the other groups (37.1 % in the metformin 500 mg group and 25.7 % in the metformin 1000 mg group), but the difference was not significant (p = 0.069) (Supplementary Table 1).
There was within-subject effect according to the time (baseline, 12, 24 weeks, or baseline, 24 weeks) in metabolic profiles and depression symptom score (Table 1). The intention-to-treat analysis results showed the metformin 1000 mg group to have a marginally significant mean decrease from baseline to end of trial in glucose and HbA1C levels relative to the change in levels in the placebo and metformin 500 mg groups (p interaction = 0.094 and 0.073, respectively). Over the trial period, insulin levels showed non-significant decreases in all groups. Moreover, weight loss, the other obesity indices, and lipid profiles were not significantly associated with metformin effects.
Our analysis of the change in glucose and HbA1c levels between baseline and end of trial showed that the metformin 1000 mg group had the greatest decline in glucose and HbA1c levels between week 0 and 6 months (p = 0.008 and 0.009, respectively) (Table 2). Patients’ BMI levels modified the metformin effect over the 0–6 months treatment period resulting in significant changes to glucose and HbA1c levels (p interaction with BMI = 0.007 and 0.0.085, respectively). Moreover, the 0–6 month glucose and HbA1c decline levels in the metformin group were modified more by the rise of BMI levels than the BMI-related modifications in the other two groups. Patients’ hormone receptor (HR) status and TMX use did not modify the metformin effect.
The types and numbers of adverse events are summarized in Supplementary Table 2. The proportion of total adverse events increased from placebo to metformin 500 mg and metformin 1000 mg groups (5.7, 14.3, and 20.0 %, respectively), but the increase was not significant (p trend = 0.08). There was one serious event (dizziness) in the metformin 500 mg group. Unexpected adverse events were reported in 2 patients in the metformin 500 mg group and 1 patient in the metformin 1000 mg group. Of the unexpected events, a new breast mass occurred in the metformin 1000 mg group, and a pleural mass was detected in the metformin 500 mg group; the tumors were Stage IIa grade 3 and Stage IIIa grade 3, respectively.
Discussion
This randomized, double-blind, placebo-controlled trial evaluated the preferential efficacy of metformin in obese, non-diabetic, breast cancer patients, and the results showed that daily treatment with 1000 mg of metformin produced favorable effects, as indicated by decreases in glucose and HbA1c levels relative to placebo and 500 mg treatments.
There have been five recent clinical trials of metformin intervention in breast cancer patients [16–20]. Although a placebo-controlled trial is necessary to compare pre- and post-treatment effects since breast cancer patients after breast surgery usually lose weight due to the operation and voluntarily change their behaviors and lifestyles toward a healthy lifestyle pattern, two trails did not use placebo group [16, 17]. Also blinding method is important to avoid information bias; however, only two trials had blinding [18, 20]. Even though one trial was single-arm trial [17], three trials were used with metformin for a short-term under 1 month [17–19]. Only one trial continued to use high-dose metformin (1000 and 1500 mg with dose escalation protocol from 500 mg) for at least 5 months [16]; however, this study had no blinding and placebo group. Our study can overcome the limitations of prior trials: we included multiple comparisons with repeated measurement among multiple groups, a blind method including placebo with identical-appearing pills, different metformin dose levels with dose escalation, and a long treatment period. Also, our trial evaluated the interaction between BMI and metformin in diabetes profiles.
Despite the effectiveness of the high dose of metformin, the adverse events increased by dose levels [18]. In our study, two patients showed a new breast and pleural mass for taking metformin, respectively. Both patients had Stage IIa and IIIa grade 3 breast cancers. Although these events were causally unrelated, all adverse events, in particular, in high-grade cancer patients should be closely monitored during postoperative adjuvant metformin therapy.
Our study showed that metformin was more effective in non-diabetic breast cancer patients with a high BMI (>27 kg/m2). Similarly, Bonanni et al. [18] reported that glucose levels were reduced by metformin treatment in non-diabetic breast cancer patients with BMI >27 kg/m2.
It is encouraging that, regardless of HR status, there was a beneficial effect of metformin on diabetes profiles in non-diabetic breast cancer patients. An in vitro study, which indicated that metformin’s role in inhibiting cell proliferation is independent of those of estrogen and HER2 receptors [21], supports our result.
Metformin has anticancer effect through insulin-dependent and non-dependent pathway [22]. Metformin activates AMPK directly resulting in inhibition of mTOR, cell growth, and proliferation. Also metformin acts on cancer cell indirectly by reducing hepatic gluconeogenesis and circulating insulin level. Insulin is a potential biologic mediator to expression of insulin/IGF-1 receptors on breast cancer cells. In an epidemiologic study, metformin significantly lowered fasting insulin levels by 22.4 % and improved insulin sensitivity by 25.6 % [11]. In our study, insulin level decreased by 14.3 % after 6 month treatment of metformin 1000 mg compared to 6.9 % decrease in the placebo group, although there was no statistical significance due to small sample size.
Further studies are needed to determine whether long-term use of metformin by breast cancer patients can be tolerated without serious adverse events, and whether such long-term metformin treatment is effective as a tertiary preventive against breast cancer recurrence and mortality. A new phase III randomized trial to define the efficacy of metformin as adjuvant treatment among early-stage breast cancer patients is in underway worldwide.
Some factors limit the conclusions that can be drawn from our clinical trial. First, this trial did not permit long-term evaluation of advantages of metformin treatment in terms of breast cancer prognosis, since it was performed over a short 6-month period. Second, the dropout population (28.6 %) in our study was relatively high. Such losses and small sample size could result in insufficient statistical power in our per-protocol analysis. Despite these limitations, the results of this randomized, double-blind, placebo-controlled study to evaluate the effects of metformin intervention on multiple parameters in non-diabetic breast cancer patients who underwent breast cancer surgery and adjuvant systemic therapy indicate that metformin use has therapeutic potential in a non-diabetic population with a specific disease history. Compared to prior clinical trials, our trial compensated for the absence of multiple within- and between-subject comparisons.
In summary, daily 1000 mg metformin treatment had a favorable effect on controlling glucose intolerance, indicating its prognostic importance. Even though we could not make firm conclusion regarding the tendency toward a dose-dependent increase in adverse events due to small sample size, further study with evaluation of causal relationship and large sample size is required. Although we could not evaluate metformin’s efficacy on prolonged survival of breast cancer patients, the results do indicate an anticancer potential of metformin, thereby improving the prognosis of breast cancer patients. Additional clinical trials with larger numbers of breast cancer patients are needed to elucidate the risk–benefit ratio of long-term use of metformin and evaluate metformin-related patient survival benefits.
References
Gonzalez-Angulo AM, Meric-Bernstam F (2010) Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res 16(6):1695–1700
Nakano M, Inui A (2012) Metformin and incretin-based therapies up-regulate central and peripheral adenosine monophosphate-activated protein affecting appetite and metabolism. Indian J Endocrinol Metab 16(Suppl 3):S529–531. doi:10.4103/2230-8210.105567
Hadad SM, Fleming S, Thompson AM (2008) Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol 67(1):1–7
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403
Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD (2009) Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 8(6):909–915
Malki A, Youssef A (2011) Antidiabetic drug metformin induces apoptosis in human MCF breast cancer via targeting ERK signaling. Oncol Res 19(6):275–285
Bowker SL, Yasui Y, Veugelers P, Johnson JA (2010) Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 53(8):1631–1637
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330(7503):1304–1305
Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, Melani C, Vitale V, Romano D, Barchielli A, Marchionni N, Rotella CM, Mannucci E (2011) Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 34(1):129–131
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL (2012) Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35(2):299–304
Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG (2008) Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer 8(6):501–505. doi:10.3816/CBC.2008.n.060
Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27(20):3297–3302. doi:10.1200/JCO.2009.19.6410
Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29(1):54–60. doi:10.1200/JCO.2010.29.3183
WHOIOTF/IASO (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Int Diabetes Inst, Melbourne
Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, He YQ, Liu YJ, Chen JD, Li LH (2008) Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 299(2):185–193
Campagnoli C, Pasanisi P, Abba C, Ambroggio S, Biglia N, Brucato T, Colombero R, Danese S, Donadio M, Venturelli E, Zito G, Berrino F (2012) Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer 12(3):175–182. doi:10.1016/j.clbc.2012.03.004
Niraula S, Dowling RJ, Ennis M, Chang MC, Done SJ, Hood N, Escallon J, Leong WL, McCready DR, Reedijk M, Stambolic V, Goodwin PJ (2012) Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 135(3):821–830. doi:10.1007/s10549-012-2223-1
Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS, Galimberti V, Veronesi P, Johansson H, Aristarco V, Bassi F, Luini A, Lazzeroni M, Varricchio C, Viale G, Bruzzi P, Decensi A (2012) Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol 30(21):2593–2600. doi:10.1200/JCO.2011.39.3769
Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder-Thompson S, Dewar JA, Thompson AM (2011) Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 128(3):783–794. doi:10.1007/s10549-011-1612-1
Goodwin PJ, Parulekar WR, Gelmon KA, Shepherd LE, Ligibel JA, Hershman DL, Rastogi P, Mayer IA, Hobday TJ, Lemieux J, Thompson AM, Pritchard KI, Whelan TJ, Mukherjee SD, Chalchal HI, Oja CD, Tonkin KS, Bernstein V, Chen BE, Stambolic V (2015) Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Nat Cancer Inst 107(3). doi:10.1093/jnci/djv006
Zhuang Y, Miskimins WK (2008) Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal 3:18. doi:10.1186/1750-2187-3-18
Goodwin PJ, Stambolic V (2011) Obesity and insulin resistance in breast cancer–chemoprevention strategies with a focus on metformin. Breast 20(Suppl 3):S31–35. doi:10.1016/S0960-9776(11)70291-0
Acknowledgments
This study was supported by a research grant from the Cancer Research Institute, Seoul National University (2008), the Education and Research Encouragement Fund of Seoul National University Hospital, and grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420190).
Authors’ contributions
All authors participated in the design, conduct, and analysis of the study and have approved the final version of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ko, KP., Ma, S.H., Yang, JJ. et al. Metformin intervention in obese non-diabetic patients with breast cancer: phase II randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat 153, 361–370 (2015). https://doi.org/10.1007/s10549-015-3519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3519-8