Abstract
MYC amplification has been reported as a prominent feature of secondary angiosarcomas (SAS). The differential diagnosis between atypical vascular lesion (AVL) and low-grade angiosarcoma (AS) can be occasionally very difficult or even impossible, and MYC amplification status has been pointed as an important diagnostic tool to distinguish cutaneous vascular lesions of the breast. We assessed MYC amplification and protein expression status by fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC), respectively, in 49 patients diagnosed with breast AS, and 30 patients diagnosed with post-radiation AVL of the breast. Clinical and pathological features, and follow-up data were collected, and survival analyses were performed. Among 37 patients with SAS, twenty patients had tumors with high-level MYC amplification and protein overexpression (54 %). None of primary angiosarcomas (PAS) or AVL cases showed MYC amplification or protein expression. Concordance between MYC amplification (FISH) and protein expression (IHC) was 100 % in AVL, PAS, and SAS. Survival analysis of the SAS patients demonstrates that those with MYC amplification had a significantly worse overall survival compared to cases without MYC amplification (P = 0.035). There was a non-significant trend toward a poor disease-free survival between cases with and without MYC amplification (P = 0.155). Our findings show that MYC amplification is a highly specific but poorly sensitive marker for SAS and, therefore, a negative result does not exclude the diagnosis of angiosarcoma. MYC amplification was associated with adverse prognosis, suggesting a prognostic role of MYC amplification status on SAS of the breast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary angiosarcoma (SAS) is an aggressive tumor that arises in the setting of previous irradiation field or chronic lymphedema (Stewart–Treves syndrome) and is associated with poor prognosis [1–3]. Atypical vascular lesions (AVL) of the breast, however, are a benign radiation-associated vascular proliferation that shows morphological similarities with low-grade secondary angiosarcomas [4–9]. The differential diagnosis between AVL and low-grade angiosarcoma (AS) can be occasionally very difficult or even impossible, mostly in small-sized skin biopsies, due to the histologic overlap characteristics between low-grade AS and AVL [5, 10]. Some studies reported patients with AVL who later developed AS, suggesting that AVL may be a precursor to or an incipient angiosarcoma [6, 7, 11].
Some studies have shown that c-myc amplification is a recurrent genetic alteration in secondary angiosarcomas, but not in primary angiosarcomas and AVL, suggesting distinct pathogenetic mechanisms between them [12–15]. However, recent studies have shown primary angiosarcomas with MYC amplification, raising the possibility of MYC participation in its pathogenesis [10, 12, 16]. MYC is a proto-oncogene that codes for a transcription factor involved in the regulation of cellular proliferation, cell growth, and apoptosis [4, 12]. The most common mechanisms by which MYC activation occurs in tumors are gene amplification and gene rearrangement [4, 10]. The deregulation of c-myc has been associated with human cancers and has also been implicated in angiogenesis [17]. MYC protein expression promotes cell proliferation through inappropriate entry to S-phase from G1 phase following ionizing radiation, resulting in its function as an oncogene [18].
As accurate morphological diagnosis between low-grade AS and AVL can be difficult and the utility of immunohistochemical (IHC) stains for this particular diagnostic dilemma has not been established [11], new methods to distinguish these two biologic entities are a welcome tool in the clinical setting.
In this study, we aim to evaluate the diagnostic utility of MYC amplification and overexpression in the scenario of AVL and AS to further assess whether MYC amplification is implicated in prognosis.
Materials and methods
We searched the database from the Department of Anatomic Pathology of the European Institute of Oncology (IEO) Milan, Italy, and Portuguese Institute of Oncology (IPO), Lisbon, Portugal, and retrieved all female patients with the diagnosis of either angiosarcoma (primary and secondary) or AVL of the breast from 1999 to 2014. Post-radiation AVL and angiosarcoma arising at sites other than the breast and cases without representative tumor blocks were not included in this study. The medical records were reviewed and hematoxylin- and eosin-stained slides were re-examined by three of the authors (CFG, SA, and HG) to confirm the diagnosis. Histopathological diagnostic criteria proposed by Fineberg and Rosen [19] were used in the pathological review. The histological grade of breast angiosarcomas was determined as previously described by Donell et al. [20] modified by Schnitt and Collins [21]. Suitable blocks were chosen to obtain additional sections for MYC immunostainings and interphase FISH analysis.
For IHC analysis, standard whole sections were immunostained for MYC with a rabbit monoclonal anti-c-MYC antibody (Y69, 1:50, Epitomics [Cat no. 1472-1], Burlingame, CA) using heat-induced epitope retrieval and an automated immunostainer (Ventana, Oro Valley, AZ, USA). Appropriate positive and negative controls were used. Only nuclear reactivity was considered positive. Immunostained sections were then examined by routine light microscopy. The cases were scored as ‘negative’ (< 5 % positive cells), ‘1+’ (5–25 % positive cells), ‘2+’ (26–50 % positive cells), or ‘3+’ (≥51 % positive cells), as previously proposed by Shon et al. [12].
Interphase FISH was performed using commercially available FISH probe for MYC (8q24), and a probe designed to detect CEP8 (Abbot Molecular, Des Plines, IL, USA). All tissue sections were pretreated, digested, and washed as recommended by the probes supplier. A minimum of 50 non-overlapping intact interphase nuclei were assessed for the presence of amplification and were analyzed by two observers blinded to the original diagnosis. The hybridized slides were reviewed and the ratio of MYC (red) and CEP8 (green) signals was calculated. MYC/CEP8 ratio of 2.0 or higher defined MYC amplification.
When comparing characteristics between primary, secondary angiosarcomas with MYC amplification and secondary angiosarcomas without MYC amplification, for categorical variables, we used the Fisher’s exact test or the Chi-square test. For continuous variables, the non-parametric median two-sample test was used. Disease-free survival (DFS) was calculated from the date of diagnosis of angiosarcoma to any local, regional, distant relapse or death from any cause, whichever occurred first, or to last visit date in case of no events. Overall survival (OS) was defined as the time interval from date of diagnosis of angiosarcoma to death from any cause or to last date of follow-up. DFS and OS were calculated with the Kaplan–Meier method and compared across different subgroups by means of the Log-rank test or Log-rank test for trend, as appropriate. Multivariable Cox regression models were used to adjust the effect of the different types of angiosarcoma on survival. Variables that were significant in the univariate analysis were tested in the multivariable models and only significant or borderline significant (P < 0.10) variables in the multivariable models were included in the final model. Hazard ratios (HR) and 95 % confidence intervals (CI) were reported. All analyses were carried out with the Statistical Analysis System (SAS) software (SAS Institute, Cary, NC) and the R (http://cran.r-project.org/) software. All the reported P values were two sided.
Informed consent was obtained from all patients and/or guardians and institutional review board approvals were obtained for all parts of the study.
Results
Clinicopathological features
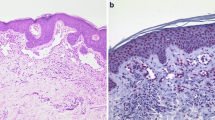
Forty-nine patients were diagnosed with breast angiosarcoma. Of these 49 patients diagnosed with breast angiosarcoma, thirty-seven patients had a previous history of breast carcinoma treated with radiation therapy (Fig. 1d), and twelve patients had a diagnosis of primary (sporadic) angiosarcoma. All patients with primary AS presented with palpable mass, without any skin changes. Patients with secondary AS presented with skin changes (rash and ecchymosis) with concomitant ulceration and/or bruising. Clinicopathological features from 28 cases of AS included in the present series were previously described [3]. Thirty patients were diagnosed with post-radiation AVL of the breast (Fig. 1a), but one patient was excluded from this study because there was no more representative tumor block available for MYC analysis. Clinicopathological details of these patients with AVL were published previously [11]. The main method used for the pathological diagnosis of AS was core biopsy (92 % of cases), and for diagnosis of AVL was punch biopsy (50 % of cases), followed by excisional biopsy. The clinicopathological characteristics of the three study groups are summarized in Tables 1, 2, and 3.
a AVL of the breast skin showing circumscribed proliferation of vessels in the upper dermis, mild endothelial atypia, and associated lymphocytic infiltration. Hematoxylin and eosin, ×200. b In a MYC-immunostained section of the same AVL, there was no nuclear staining in endothelial cells. c FISH for MYC amplification showing no MYC amplification in AVL. d Secondary AS with epithelioid morphology showing proliferation of atypical vessels. Hematoxylin and eosin, ×200. e Secondary AS showing nuclear MYC protein expression in proliferative tumor cells.f FISH analysis showed high-level MYC gene amplification in the secondary AS
Immunohistochemical data
Immunohistochemical stains for MYC protein were performed on all cases of AVL and AS. None of the 29 cases of AVL displayed nuclear immunoreactivity for MYC (Table 1, Fig. 1b). Twenty specimens of secondary angiosarcoma (54 %) stained positive for c-myc (20/37 cases = 54 %), with strong positive staining (‘3+’ or >51 % positive) observed in 10 cases (10/20 cases = 50 %) (Table 3; Fig. 1e). None of the primary AS cases showed MYC protein expression (Table 2).
Fluorescence in situ hybridization (FISH)
None of the 29 cases of AVL (Fig. 1c) or PAS showed MYC amplification (Tables 1, 2). Twenty out of 37 cases (54 %) of secondary AS showed MYC amplification using interphase FISH analysis (Table 3; Fig. 1f). Of 3 patients with secondary angiosarcoma with epithelioid features, 2 cases showed MYC amplification, and MYC protein overexpression.
Concordance between FISH and IHC
Concordance between protein expression (IHC) and MYC amplification was 100 % in AVL and primary and secondary AS. The two cases of AVL that progressed to AS (Cases 22 and 23, Table 1) showed no MYC amplification or protein overexpression both at diagnosis of AVL, as at the time of diagnosis of AS (Cases 4 and 11, Tables 3, 4).
MYC amplification/protein overexpression as a prognostic factor
Clinical follow-up data of patients with diagnosis of breast angiosarcoma were available for 48 of the 49 patients (median 32 months, range from 1 to 163 months).
There was no correlation between histological tumor grade and MYC amplification (P = 0.365). No significant association of tumor size and MYC amplification was found (P = 0.289).
When comparing DFS between primary AS, secondary AS without MYC amplification and secondary AS with MYC amplification, there were statistically significant differences at both univariate and multivariable analyses (P = 0.033, Fig. 2; Table 5; P = 0.016; Table 6). When comparing OS between primary AS, secondary AS without MYC amplification and secondary AS positive for MYC amplification, there were statistically significant differences only at multivariable analyses (P = 0.084, Fig. 3; Table 5; P = 0.012; Table 6). When limiting the survival analysis to the secondary AS patients, we observed that cases with MYC amplification had a significantly worse OS compared to cases without MYC amplification (size and grade-adjusted HR: 3.47 (1.09–11.1)). There was a non-significant trend toward a poor DFS between cases with and without MYC amplification (size and grade = adjusted HR: 1.89 (0.78–4.55), Table 7).
Other disease parameters (tumor size and tumor grade) were also independently predictive of worse outcome in both primary and secondary angiosarcomas. Patients that presented tumor size >5 cm had a short-term DFS (P = 0.054) and poor OS (P = 0.020) when compared with cases showing tumor size ≤5 cm in multivariable analysis (Table 6). High-grade tumors were associated with worse DFS and OS (P = 0.056 and P = 0.018, respectively; Table 6) when compared with low-grade tumors.
MYC amplification, however, had no significant association with a shorter latency period (time from radiation therapy to the diagnosis of secondary angiosarcoma) in cases with versus in cases without MYC amplification (P = 0.161).
Discussion
MYC amplification has been described in different solid tumors. MYC high-level amplification has been reported as a prominent feature of radiation-induced angiosarcomas and is also prevalent in other radiation-induced sarcomas, suggesting a strong association between irradiation and MYC gene amplification [22]. The perspective of MYC as an important anticancer target determines the importance to understand the specific role of MYC in different subsets of sarcomas [23, 24].
Our study evaluated the largest series of MYC amplification in the largest series of primary and secondary angiosarcomas and AVL of the breast, and it is the unique study that explored exclusively vascular lesions occurring within the breast. In our study, MYC amplification was detected in 54 % of secondary breast angiosarcomas. Two other studies found 55 and 67 % of MYC amplification in secondary angiosarcomas, respectively [14, 16]. We did not find MYC amplification or protein overexpression in any case of AVL or primary angiosarcoma of the breast, and our results confirm previous findings of other series [5, 10, 13–15].
Some studies, however, have found that a high level of amplification of MYC on chromosome 8q24-21 is present in 100 % of the patients with post-radiation angiosarcoma and lymphedema-associated angiosarcoma, but not in AVL and primary angiosarcoma [5, 10, 13, 15], suggesting MYC analysis as a crucial diagnostic tool in the setting of vascular lesions. All these studies, however, involved small case series. They also studied cases of angiosarcoma from non-mammary sites and lymphedema-associated angiosarcoma all together, and this fact may represent a selection bias. Kacker et al. found 58 % frequency of MYC high-level amplifications in their series of radiation-induced angiosarcomas, including sarcomas of the breast but they also included AS of other organs. When only AS of the breast was counted, the frequency of MYC high-level amplifications was 86 % [22].
Based on our findings, the diagnostic usefulness of interphase FISH and MYC immunohistochemistry in distinguishing low-grade secondary angiosarcoma from AVL is limited due to the low sensitivity of these assays. Indeed, a negative result does not exclude the diagnosis of angiosarcoma.
In our series, none of the 12 primary angiosarcoma of the breast showed MYC amplification. Recent studies, however, have shown a small subset of primary angiosarcoma that also presents MYC amplification, suggesting that genomic amplification of MYC is not restricted to secondary AS, as previously recognized [10, 12, 16]. Shon et al. detected MYC abnormalities in a small number of primary cutaneous angiosarcomas, but they included male and female patients with angiosarcomas from non-mammary skin [12]. Italiano et al. found MYC amplification in three out of the six primary cases of angiosarcoma (2 out of the 3 cases occurring within the breast) [16]. It seems that the absence of high-level gene amplifications does not exclude a possible role of MYC in the pathogenesis of primary angiosarcomas. We can also hypothesize that MYC amplification is not a specific genomic aberration induced by ionizing radiation in secondary angiosarcomas, as MYC amplification has also been shown even in lymphedema-associated angiosarcomas [10, 13–15].
Two patients of our series with initial diagnosis of AVL showed progression to high-grade cutaneous angiosarcoma (Cases 22 and 23, Table 1). Patient of case 22 developed a high-grade breast AS 19 months after diagnosis of AVL in the exact same location of previous punch biopsy. Patient of case 23 developed a bilateral high-grade epithelioid AS 89 months after the initial diagnosis of AVL in the previous punch biopsy site. Details of these cases were previously described [11]. Both at diagnosis of AVL, as at the time of diagnosis of AS, no MYC amplification and protein overexpression were observed. Santi et al. found a common mutational pathway (mutational inactivation of TP53 gene) among AVL and AS, suggesting that they are biologically related entities and could represent the extremes of a morphological continuum [25]. Most studies that explored MYC amplification status cast doubt on this hypothesis, since, to date, no case of MYC amplification in AVL has been identified [13, 15]. However, based on our findings, the hypothesis that AVL may represent a precursor lesion should not be discarded based only on MYC amplification status, since not all cases of post-radiation angiosarcoma shows MYC amplification.
We also found excellent FISH and IHC concordance in primary and secondary angiosarcomas, and AVL of the breast, confirming previous studies [5, 13]. Different from our results, Ginter et al. found a poor concordance (65 %) between IHC and FISH for MYC in AS of non-mammary sites [10]. Shon et al. reported MYC protein overexpression in cases lacking gene amplification in primary cutaneous angiosarcomas, suggesting other mechanisms of MYC activation, but they also included cases of AS from other organs [12]. Therefore, we can conclude that MYC amplification and protein overexpression in angiosarcomas are highly specific but low sensitive marker for the diagnosis of angiosarcomas of the breast and other non-mammary sites.
Our findings confirm the fact that secondary tumors have a worse prognosis when compared with primary disease, as we previously reported in a series addressing a smaller number of cases [3, 26]. We also found a significant association between MYC amplification and poor prognosis in secondary angiosarcomas of the breast. Previous studies have shown an association between gene amplification and/or protein overexpression of MYC and advanced stage in a variety of non-angiosarcoma human malignancies [12, 27], but none of the available studies that explored the prognostic role of MYC gene in angiosarcomas was able to find any association between MYC amplification and clinical prognosis or tumor grade [12, 14, 16, 22].
Our study is the first one to demonstrate a consistent association between MYC amplification status and clinical outcome on secondary angiosarcoma. We did not find any association between MYC amplification and tumor size, tumor grade or shorter latency period from radiation therapy, and diagnosis of AS. To date, only the study of Kacker et al. found a non-significant statistical trend toward a shorter latency between primary tumor and sarcoma in cases with MYC amplification (P = 0.2).
Data from current literature and our results demonstrate that the genetic and molecular aberrations involved in AS tumorigenesis remain poorly understood. The genetic heterogeneity of these tumors has been observed by other authors, and new potential genomic events have been investigated. Guo et al. identified FLT4 gene coamplification with MYC in 25 % of secondary angiosarcomas, but none of AVL and primary AS showed this abnormality [15]. Italiano et al. observed that the NOTCH pathway effector gene MAML1 (5q35.3) is amplified and overexpressed in 18 % of secondary angiosarcomas, in all these cases, coamplified with FLT4. They did not find any difference in clinical or pathologic characteristics between AS with and without 5q35 amplification [16].
Radiation-induced AS seems to be genetically different from primary angiosarcomas and AVL, but there is a clear evidence of genetic heterogeneity even among secondary cases. Therefore, further studies are necessary to identify the oncogenic trigger events of this subset of tumors.
References
Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW (2004) Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol 28(6):781–788
Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A (2005) Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer 104(4):856–863. doi:10.1002/cncr.21223
Fraga-Guedes C, Gobbi H, Mastropasqua MG, Botteri E, Luini A, Viale G (2012) Primary and secondary angiosarcomas of the breast: a single institution experience. Breast Cancer Res Treat 132(3):1081–1088. doi:10.1007/s10549-011-1931-2
Feller JK, Mahalingam M (2013) c-myc and cutaneous vascular neoplasms. Am J Dermatopathol 35(3):364–369. doi:10.1097/DAD.0b013e31827aad83
Fernandez AP, Sun Y, Tubbs RR, Goldblum JR, Billings SD (2011) FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol 39(2):234–242. doi:10.1111/j.1600-0560.2011.01843.x
Patton KT, Deyrup AT, Weiss SW (2008) Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol 32(6):943–950
Brenn T, Fletcher CD (2005) Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol 29(8):983–996
Mattoch IW, Robbins JB, Kempson RL, Kohler S (2007) Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol 57(1):126–133. doi:10.1016/j.jaad.2006.10.025
Lucas DR (2009) Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med 133(11):1804–1809. doi:10.1043/1543-2165-133.11.1804
Ginter PS, Mosquera JM, MacDonald TY, D’Alfonso TM, Rubin MA, Shin SJ (2013) Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol 45(4):709–716. doi:10.1016/j.humpath.2013.11.002
Fraga-Guedes C, Gobbi H, Mastropasqua MG, Rocha RM, Botteri E, Toesca A, Viale G (2014) Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat 146(2):347–354. doi:10.1007/s10549-014-3020-9
Shon W, Sukov WR, Jenkins SM, Folpe AL (2014) MYC amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in situ hybridization and immunohistochemical study. Mod Pathol 27(4):509–515. doi:10.1038/modpathol.2013.163
Mentzel T, Schildhaus HU, Palmedo G, Buttner R, Kutzner H (2012) Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol 25(1):75–85. doi:10.1038/modpathol.2011.134
Manner J, Radlwimmer B, Hohenberger P, Mossinger K, Kuffer S, Sauer C, Belharazem D, Zettl A, Coindre JM, Hallermann C, Hartmann JT, Katenkamp D, Katenkamp K, Schoffski P, Sciot R, Wozniak A, Lichter P, Marx A, Strobel P (2010) MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 176(1):34–39. doi:10.2353/ajpath.2010.090637
Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR (2011) Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 50(1):25–33. doi:10.1002/gcc.20827
Italiano A, Thomas R, Breen M, Zhang L, Crago AM, Singer S, Khanin R, Maki RG, Mihailovic A, Hafner M, Tuschl T, Antonescu CR (2012) The miR-17-92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer 51(6):569–578. doi:10.1002/gcc.21943
Pelengaris S, Khan M, Evan G (2002) c-MYC: more than just a matter of life and death. Nat Rev Cancer 2(10):764–776. doi:10.1038/nrc904
Sheen JH, Dickson RB (2002) Overexpression of c-Myc alters G(1)/S arrest following ionizing radiation. Mol Cell Biol 22(6):1819–1833
Fineberg S, Rosen PP (1994) Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol 102(6):757–763
Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr, Kinne DW (1981) Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 5(7):629–642
Schnitt SJ, Collins LC (2009) Vascular lesions. In: Jr JP (ed) Biopsy interpretation of the breast, 1st edn. Lippincott Williams & Wilkins, Philadelphia, pp 344–360
Kacker C, Marx A, Mossinger K, Svehla F, Schneider U, Hogendoorn PC, Nielsen OS, Kuffer S, Sauer C, Fisher C, Hallermann C, Hartmann JT, Blay JY, Mechtersheimer G, Hohenberger P, Strobel P (2013) High frequency of MYC gene amplification is a common feature of radiation-induced sarcomas. Further results from EORTC STBSG TL 01/01. Genes Chromosomes Cancer 52(1):93–98. doi:10.1002/gcc.22009
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146(6):904–917. doi:10.1016/j.cell.2011.08.017
Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM, Karnezis AN, Swigart LB, Nasi S, Evan GI (2008) Modelling Myc inhibition as a cancer therapy. Nature 455(7213):679–683. doi:10.1038/nature07260
Santi R, Cetica V, Franchi A, Pepi M, Cesinaro AM, Miracco C, Paglierani M, De Giorgi V, Delfino C, Difonzo EM, Pimpinelli N, Bianchi S, Sardi I, Santucci M, Massi D (2011) Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology 58(3):455–466. doi:10.1111/j.1365-2559.2011.03770.x
Toesca A, Spitaleri G, De Pas T, Botteri E, Gentilini O, Bottiglieri L, Rotmentsz N, Sangalli C, Marrazzo E, Cassano E, Veronesi P, Rietjens M, Luini A (2012) Sarcoma of the breast: outcome and reconstructive options. Clin Breast Cancer 12(6):438–444. doi:10.1016/j.clbc.2012.09.008
Nesbit CE, Tersak JM, Prochownik EV (1999) MYC oncogenes and human neoplastic disease. Oncogene 18(19):3004–3016. doi:10.1038/sj.onc.1202746
Acknowledgments
This work was supported partially by Grants from Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo a Pesquisa de São Paulo (FAPESP), Brazil; European Institute of Oncology, Italy, and Portuguese Institute of Oncology of Lisbon, Portugal. This research complies with the Brazilian, Italian, and Portuguese current laws. The authors declare that they do not have financial relationship with the organizations that sponsored this research.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraga-Guedes, C., André, S., Mastropasqua, M.G. et al. Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res Treat 151, 131–140 (2015). https://doi.org/10.1007/s10549-015-3379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3379-2