Abstract
The 21-gene recurrence score (RS) assay (Oncotype DX™) predicts the likelihood of breast cancer recurrence and chemotherapy responsiveness. The aims of this study were to describe temporal trends in assay usage, to investigate factors associated with the receipt of the assay and to determine how the assay is associated with treatment decisions. Random samples of stage I–II female breast cancer patients diagnosed in 2004, 2005 and 2010 as reported to the National Cancer Institute’s Surveillance Epidemiology and End Results program were included. Among women diagnosed in 2010 with estrogen receptor positive (ER+), lymph node-negative (LN−) tumors, factors associated with receipt of the assay were identified and the likelihood of chemotherapy by RS was estimated. Assay usage increased over time (ER+/LN−:8.0–27.0 %, p < 0.01; ER+/LN+: 2.0–15.7 %, p = 0.09; ER−: 0.2–1.7 %, p < 0.01) from 2005 to 2010. Receipt of the assay was associated with younger age, lower area income and tumor characteristics. Among women in the low (RS < 18) and high risk (RS > 30) categories, 3.3 and 95.9 % received chemotherapy, respectively. Within the intermediate risk group the receipt of chemotherapy varied: 12.8 % (RS: 18–19), 35.0 % (RS: 20–23) and 84.0 % (RS: 24–30). During the study years, assay usage increased among women for whom the assay is and is not guideline recommended. Factors such as insurance and race/ethnicity do not appear to be associated with the receipt of the assay. The RS, as determined broadly via three categories and within the intermediate risk group, does appear to influence chemotherapy decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of women diagnosed with breast cancer in the United States have early stage, estrogen receptor-positive (ER+) tumors [1]. The primary treatment for these tumors consists of mastectomy or breast-conserving surgery with radiation, followed by systemic therapy. Although approximately 85 % of women with early-stage ER+ tumors treated with only hormone therapy following surgery were shown to be recurrence-free at 10 years, [2] historically, chemotherapy has also been recommended [3]. Many female breast cancer patients, therefore, may have experienced substantial side-effects while gaining minimal benefit from receiving chemotherapy.
Genetic profiling assays are increasingly being used to identify breast cancer subtypes that are more likely to recur and/or are more responsive to chemotherapy. This allows women with subtypes that are considered less likely to recur or less responsive to chemotherapy to be spared from receiving chemotherapy and its potential toxicities. The most common genetic profiling test for breast cancer in the United States is the 21-gene recurrence score assay (Oncotype DX™, Genomic Health, Inc.), which is a reverse-transcriptase polymerase chain reaction (RT-PCR) assay that predicts 10-year distant recurrence risk based on the expression of 21 genes [4]. The resulting recurrence score, which is a continuous predictor between 0 and 100, is used to categorize women into low, intermediate, and high recurrence risk groups. The test was initially validated to predict the risk of recurrence and response to chemotherapy among women with ER+, lymph node-negative (LN−) tumors who received hormone therapy [2, 5, 6].
Shortly after the assay was commercially introduced in the United States in 2004, the American Society for Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) issued recommendations for its use among women with ER+/LN− breast cancers [7, 8]. Clinical treatment recommendations were also issued for these women based on assay results; the NCCN recommends hormone therapy alone for the low-risk category, hormone therapy with or without chemotherapy for the intermediate risk category and hormone therapy with chemotherapy for the high risk category [8]. Although there are some data to suggest that the 21-gene recurrence score may predict recurrence and chemotherapy response among women with ER+/LN+ tumors, [9–11] guidelines have yet to be expanded to include recommendations for other ER/LN subgroups. An ongoing clinical trial (RxPONDER) led by the Southwest Oncology Group (SWOG), and member of the National Clinical Trials Network is addressing this question [12].
Previous studies have documented an increase in usage of the 21-gene recurrence score assay over time, [13, 14] but none have quantified national, population-based temporal trends by ER/LN status to assess guideline adherence. Previous studies have also investigated factors associated with the receipt of the test but have been conducted among select breast cancer patients (e.g., by geographic location, [14, 15] healthcare insurer, [13] or place of care [14, 16, 17]). The recurrence score has also been shown to impact broad clinical decision making, [18] but it remained unclear how the recurrence score impacts treatment decisions among women categorized to the intermediate-risk group [16, 19]. Therefore, the aims of this study were to describe the temporal trends in 21-gene recurrence score assay usage based on a population-based sample from areas across the nation and among women who are recommended to have the assay to investigate factors associated with the receipt of the test and determine how the recurrence score is associated with treatment decisions, particularly among women in the intermediate-risk group.
Methods
Data source
Data from the National Cancer Institute’s (NCI’s) annual Patterns of Care (POC) studies were included. The POC studies evaluate the diffusion of cancer therapies across the United States by randomly sampling patients diagnosed with select cancers and ascertained through participating Surveillance, Epidemiology, and End Results (SEER) registries. Female breast cancer was selected as a cancer site in 2004, 2005, and 2010. During the selected calendar years, women with breast cancer were first reported to the SEER program, which consists of multiple population-based registries that collect data on incident cancer arising within specified geographic regions across the nation [20]. For each year, a random sample of the reported female breast cancer cases was then selected for inclusion in the annual POC study; a subset of these POC cases (as described below) were included in the current analyses. Medical records were then re-abstracted for information on demographics, date of diagnosis, tumor characteristics (e.g., ER status and 21-gene recurrence score) and cancer treatment. Treating physicians were also contacted to verify 21-gene recurrence score results and cancer treatments. Each SEER registry obtained institutional review board approval, as required, prior to initiating the POC study.
Study population
In 2004, women were eligible for inclusion in the POC study if they were diagnosed with stage I–II, ER+/LN− breast cancer and sampling was conducted by age and race/ethnicity. In 2005 and 2010, women were eligible for inclusion in the POC study if they were diagnosed with stage I–IIIA tumors regardless of ER/LN status, and were sampled by stage, age (<50 or >50) and race/ethnicity. Women were not eligible for inclusion in a POC study during any year if they had a history of cancer, except non-melanoma skin cancer, or if they were diagnosed at autopsy or via death certificate only. The current analyses were restricted to women who were diagnosed with stage I–II tumors with known ER/LN status: 2004 (n = 1089), 2005 (n = 781), and 2010 (n = 714).
Variables of interest
The 21-gene recurrence score was categorized as low (<18), intermediate (18–30), or high (>30), which were the same categories used in the validation studies [2]. For some women actual test values were not available but an indicator was; in such instances, the women were categorized according to the indicator (e.g., low/intermediate/high). Within the intermediate-risk group, the recurrence scores were further categorized into tertiles based on the distribution of women in this category.
Treatment-related variables were determined based on combined data from hospital medical record abstraction and treating physician verification. Receipt of surgery-radiation was defined as breast-conserving surgery with no/unknown radiotherapy, breast-conserving surgery with radiotherapy, and mastectomy with or without radiotherapy. After coding all chemotherapy agent(s) administered, a binary chemotherapy variable (yes/no within 6 months of diagnosis) was created.
Tumor stage was classified according to the American Joint Committee on Cancer 7th edition [21]. Human epidermal growth factor receptor-2 (HER2) was determined by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH); results were categorized as negative (IHC: 0 or 1+; FISH: <1.8), equivocal (IHC: 2+; FISH: 1.8–2.2) and positive (IHC: 3+; FISH: >2.2). If IHC and FISH results were conflicting, precedence was given to the FISH result, unless the IHC result was positive. For some women actual test values were not included in their medical records but an indicator was; in such instances, the women were categorized according to the indicator (e.g., positive/negative/equivocal, unknown). HER2 status was assessed as a covariate in this analysis because in more recent years the NCCN has refined their recommendations to include testing only among women whose tumors are also HER2-negative [8].
All comorbid conditions listed in the medical record at the hospital where the most definitive treatment was received were recorded and centrally coded at NCI. The Charlson comorbidity index score was then calculated, excluding breast cancer [22].
Demographic characteristics (age, race/ethnicity and marital status) were determined based on hospital medical records; if ethnicity was unavailable the North American Association of Central Cancer Registries Hispanic Identification Algorithm was used [23]. Patient-level insurance status (private/military, Medicare only, any Medicaid/no insurance/unknown) was determined according to the hospital medical record at the time of the most definitive procedure. Patient-level data on income are not collected by the SEER program; instead median family income (“income”) in the census tract where the patient lived at diagnosis was used as a proxy, based on the 2000 Census data. Income was then categorized into four groups (<40,000; 40,001–50,000; 50,001–60,000; >60,000).
Characteristics of the environment within which health care is delivered may impact utilization. Therefore, based on data from the American Hospital Association Annual Survey of Hospitals, [24] hospital bed size, hospital ownership (government, non-government), and presence of an approved residency training program at the hospital where the patient had their most definitive treatment were assessed as covariates.
Statistical analysis
In order to obtain estimates that reflected all eligible female breast cancers diagnosed within the SEER program by diagnosis year, sample weights, defined as the inverse of the sampling proportion for each sampling stratum, were applied. African Americans, Hispanics, Asian Pacific Islanders (APIs) and American Indians/Native Alaskans were oversampled. To account for the sample design and to correctly calculate the standard errors, all analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC) and SAS-callable SUDAAN (version 10.0.1; Research Triangle Institute, Research Triangle Park, NC).
The weighted percentage of women who received the 21-gene recurrence score assay during each study year by ER/LN status was calculated. Among women for whom the 21-gene recurrence score assay is guideline recommended (ER+/LN−), there were few women who received the 21-gene recurrence score assay in 2004 (n = 16/1089) and 2005 (n = 28/357). Therefore, these women were not included in subsequent analyses. Additionally, because guidelines do not recommend the use of the assay among women with ER− or LN+ tumors, women with these tumor types were also excluded from subsequent analyses. Factors that were associated (p < 0.15) with the receipt of the 21-gene recurrence score in bivariate Chi square tests were included in a multivariate logistic regression model in order to identify independent associations. Race/ethnicity was also included in the regression model because it was considered a theoretical confounder and thus a variable of interest. All tests were two sided and statistical significance was assessed using an alpha of 0.05.
Results
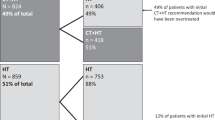
Among women diagnosed with ER+/LN− breast tumors, the use of the 21-gene recurrence score assay (Oncotype DX™) increased from 2.7 % in 2004, to 8.0 % in 2005 to 27.0 % in 2010 (p < 0.01). The use of the 21-gene recurrence score assay increased among women with ER+/LN+ tumors from 2.0 % in 2005 to 15.7 % in 2010 (p = 0.09). Usage among women with ER− tumors also increased from 0.2 % to 1.7 % (p < 0.01).
Subsequent analyses were restricted to women who were diagnosed with ER+/LN− tumors in 2010, given that the 21-gene recurrence score assay was initially only recommended for this subgroup of women [7, 8] and because this was the most current year of available data. Bivariate analyses indicated that women who were younger, resided in lower income areas and had stage II tumors were significantly more likely to receive the 21-gene recurrence score assay (Table 1). Receipt of the 21-gene recurrence score assay did not differ significantly by race/ethnicity, marital status, insurance status, hospital characteristics, existence of an approved residency program, Charlson comorbidity index or the type of surgery/radiation received and was borderline significantly associated with HER2 status.
In multivariate analysis, older age [age 70+ vs. <50: odds ratio (OR): 0.25, 95 % confidence interval (CI) 0.08–0.81] and HER2+ status (HER2+ vs. HER2−: OR: 0.21, 95 % CI 0.05–0.85; Table 1) were associated with not receiving the 21-gene recurrence score assay. Additionally, in comparison to areas with a median income level >$60,000, women with a median income between $40,000 and $50,000 were more likely to receive the 21-gene recurrence score assay (OR 5.26), but a significant association was not seen for those with a median income between $50,000 and $60,000 or less than $40,000.
Among women who received the 21-gene recurrence score assay, results were highly correlated with receipt of chemotherapy. Chemotherapy was administered to only 3.3 % of women with a low recurrence score, in comparison to 95.9 % of women with a high recurrence score (Table 2). Among women with an intermediate recurrence score, 33.2 % received chemotherapy. Additionally, within the intermediate-risk group the receipt of chemotherapy was positively associated with specific recurrence score. The receipt of chemotherapy increased from 12.8 % among women with a recurrence score of 18–19 to 84.0 % among women with a recurrence score of 24–30 (Table 2). Among women who did not have chemotherapy, the median recurrence score was 18 compared to 23 among women who did have chemotherapy.
Discussion
Although usage of the 21-gene recurrence score assay (Oncotype DX™) increased significantly from 2004 to 2010 among women with stage I–II, ER+/LN− tumors, in 2010 only roughly a quarter of these women had the assay. Additionally, even though there were no recommendations for women with tumors of other ER/LN statuses increased usage of the assay among these women was also observed, particularly among women with ER+/LN+ tumors. The receipt of the 21-gene recurrence score assay appeared to be inversely associated with age and HER2 positive status. The 21-gene recurrence score categorized both as low/intermediate/high and as three subgroups within the intermediate category was also highly correlated with receipt of chemotherapy.
Usage of the 21-gene recurrence score assay more than doubled between 2005 and 2010, which was most likely due to natural uptake of a new assay and changes in insurance coverage. In early 2006, Medicare began covering costs associated with the 21-gene recurrence score assay for women with ER+/LN− tumors if administered within 6 months of diagnosis and was intended to inform treatment decisions [25]. Subsequently, other insurers, including Aetna and United Healthcare, began covering costs associated with the assay [26–30].
Even though temporal increases were observed, based on professional guidelines, our results indicate that there is the potential for more women to benefit from the 21-gene recurrence score assay. By 2010, only a quarter of women recommended by guidelines to have the assay actually had the assay. Consistent with previous studies, [14–17] there were indications that younger age was associated with an increased likelihood of receiving the assay. However, in contrast to some [13–15, 17] by not all [16] previous studies, the current study found a higher likelihood of receiving the assay among women with more advanced tumors. There was also an indication in the current study that receipt of the assay varied by residential area income level. However, income was not assessed at the individual level and the association between receipt of the assay and area income level was non-linear. Therefore, caution should be taken when interpreting these results. Receipt of the assay was not shown to vary by race/ethnicity or insurance status, which was surprising given the cost of the test. However, most insurance carriers in the United States cover the expense of the assay for women with early-stage ER+/LN− tumors. Genomic Health Inc., the maker of the 21-gene recurrence score assay, also offers and promotes a comprehensive financial assistance program [31]. Thus, the financial burden of the assay to the breast cancer patient may be less than expected, especially among the included women who all had ER+/LN− tumors, which is the subgroup for whom the assay is widely recommended. Receipt of the assay was not shown to vary by hospital bed size, hospital ownership or hospital residency program status, which indicates that these health system factors have had little influence on the uptake of this assay. However, we cannot rule out the possibility that other unmeasured system factors, such as hospital or physician volume, may prove to be related to the receipt of the assay. Similar to previous studies [18] there was a high correlation between recurrence score and receipt of chemotherapy as recommended for women in the low and high risk categories. The current results further indicate that within the intermediate-risk group a women’s recurrence score also informs treatment decisions. Chemotherapy was administered to 84 % of women with recurrence scores 24–30 but to only 35 % of women with recurrence scores 20–23. Although it cannot be ruled out that this variation was due to confounding because small sample size precluded multivariate analyses, these findings seem to imply a strong perception that chemotherapy is beneficial among women with recurrence scores 24–30, despite there being no supporting clinical trial evidence [5]. This perception may have resulted and/or been bolstered because further assessment of the benefits from chemotherapy have focused on women with even lower recurrence scores. The Trial Assessing Individualized Options for Treatment (TAILORx) for breast cancer is a randomized Phase III trial that began recruitment in 2006 and aims to better clarify the benefit of chemotherapy and risk stratification among women with recurrence scores 11–25 [32]. Results from this trial are not expected until late 2017 [33] and will by design not be able to quantify the benefits of chemotherapy among women with recurrence scores 26–30.
This study had strengths, namely that it was population-based, oversampled minority groups, and had physician-verified 21-gene recurrence score assay results and treatment. This study also had limitations. Namely, we were not able to control for all factors that might have influenced the decisions to have the 21-gene recurrence score assay and chemotherapy, but we were able to investigate assay usage and chemotherapy patterns among female patients representative of those seen in community practice. Individual level income information was not available, therefore, caution must be taken when interpreting the observed association between receipt of the assay and census tract income level. Small sample size, especially after stratification by recurrence score, also precluded the ability to identify factors associated with the receipt of chemotherapy among women categorized to the intermediate-risk group. Receipt of other genomic assays, such as 70-gene MammaPrint assay, was not assessed; therefore, we cannot exclude the possibility that some women who were categorized as not receiving the 21-gene recurrence score assay may have received another genomic assay. The 21-gene recurrence score assay is, however, the only assay that is included in the NCCN and ASCO treatments guidelines, which is why we focused solely on the receipt of this assay. Finally, because breast cancer was not selected as a POC study cancer site since 2010 it was not possible to assess more recent trends.
In conclusion, the 21-gene recurrence score assay usage has increased among women with early-stage ER+/LN− tumors in accordance to NCCN and ASCO recommendations but usage has also increased among where there is no recommendation for use. Factors such as insurance and race/ethnicity do not appear to be associated with the receipt of the 21-gene recurrence score assay. The recurrence score, both broadly defined via three categories and within the intermediate-risk group, does appear to influence chemotherapy decisions.
References
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000–2010) Katrina/Rita population adjustment: linked to county attributes—total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. Released on April 2013, Submitted on November 2012
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ Jr, Deshler A, Fulton S, Hendricks CB, Kemeny M, Kornblith AB et al (2001) National institutes of health consensus development conference statement: adjuvant therapy for breast cancer. J Natl Cancer Inst 93:979–989
Baker J (2007) Genomic health, Inc. Pharmacogenomics 8(4):397–399
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Toi M, Iwata H, Yamanaka T, Masuda N, Ohno S, Nakamura S, Nakayama T, Kashiwaba M, Kamigaki S, Kuroi K (2010) Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer 116(13):3112–3118
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312
National Comprehansive Cancer Network. NCCN Clinical practice guidelines in oncology: breast cancer. V 1.2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast Accessed Nov 2013
Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE et al (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26:4063–4071
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28:1829–1834
ClinicalTrials.gov. Tamoxifen citrate, Letrozole, anastrozole, or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. http://clinicaltrials.gov/ct2/show/NCT01272037?term=RxPONDER&rank=1 Accessed May 2014
Haas JS, Liang SY, Hassett MJ, Shiboski S, Elkin EB, Phillips KA (2011) Gene expression profile testing for breast cancer and the use of chemotherapy, serious adverse effects, and costs of care. Breast Cancer Res Treat 130(2):619–626
Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, Hudis CA, Marcom PK, Pettinga JE, Share D et al (2012) Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 30(18):2218–2226
Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, Hearn S, Zelnak A, Styblo T, O’Regan RM (2012) 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer 118(3):788–796
Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA (2013) Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract/Am Soc Clin Oncol 9(4):182–187
DeFrank JT, Salz T, Reeder-Hayes K, Brewer NT (2013) Who gets genomic testing for breast cancer recurrence risk? Public Health Genom 16(5):215–222
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Kelly CM, Krishnamurthy S, Bianchini G, Litton JK, Gonzalez-Angulo AM, Hortobagyi GN, Pusztai L (2010) Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer 116(22):5161–5167
National Cancer Institute. SEER registry groupings for analyses. http://seer.cancer.gov/registries/terms.html Accessed 05/07/2013
Breast (2010) In: Edge SB, Byrd DR, Compton CC et al., (eds) AJCC cancer staging manual 7th ed, Springer, New York, pp 347–376. http://www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional/page3
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
National Cancer Institute. Surveillance, epidemiology, and end results program (2014) Race recode changes. http://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/ Accessed Aug 2014
American Hospital Association: American Hospital Association Annual Survery Database. http://www.aha.org/research/rc/stat-studies/data-and-directories.shtml
Genomic Health (2014) Press release: medicare contractor establishes reimbursement coverage policy for genomic health’s Oncotype DX(TM) breast cancer test. http://investor.genomichealth.com/releaseDetail.cfm?releaseID=184309 Accessed Aug 2014
Genomic Health (2014) Press release: genomic health announces national agreement with Aetna. http://investor.genomichealth.com/releasedetail.cfm?ReleaseID=219910 Accessed Aug 2014
BlueCross BlueShield of Mississippi (2014) Assays of genetic expression in tumor tissue as a technique to determine prognosis in patients with breast cancer. https://www.bcbsms.com/index.php?q=provider-medical-policy-search.html&action=viewPolicy&path=%2Fpolicy%2Femed%2FAssays_of_Genetic_Expression_in_Tumor_Tissue_as_a_Technique_to_Determine_Prognosis_in_Patients_with_Breast_Cancer.html Accessed Aug 2014
BlueCross BlueShield of North Carolina (2014) Assays of genetic expression to determine prognosis of breast cancer. http://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/assays_of_genetic_expression_to_determine_prognosis_of_breast_cancer.pdf Accessed Aug 2014
UnitedHealthcare Medicare Advantage Plans (2014) Coverage summary: genetic testing. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/UnitedHealthcare%20Medicare%20Coverage/Genetic_Testing_SH_Ovations.pdf. Accessed Aug 2014
UnitedHealthcare. Molecular pathology/molecular diagnostics/genetic testing. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Main%20Menu/Tools%20&%20Resources/Policies%20and%20Protocols/Medicare%20Advantage%20Reimbursement%20Policies/M/MolecularGeneticTest_01252013.pdf. Accessed Aug 2014
Genomic Health, Inc. (2013) Oncotype Dx FAQs: http://www.mybreastcancertreatment.org/en-US/AboutOncotypeDX/OncotypeDXFAQs.aspx#fbac3992-b9cd-489a-a325-9e84bb78f9af Accessed Dec 2013
Zujewski JA, Kamin L (2008) Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol 4(5):603–610
ClinicalTrials.gov. (2013) Hormone therapy with or without combination chemotherapy in treating women who have undergone surgery for node-negative breast cancer (The TAILORx Trial) http://clinicaltrials.gov/show/NCT00310180 Accessed Dec 2013
Acknowledgments
This work was supported by National Cancer Institute contracts: HHSN261201000024C; HHSN261201000025C, HHSN261201000032C, HHSN261201000027C, HHSN261201000026C, HHSN261201000140C, HHSN261201000037C, HHSN261201000033C, HHSN261201000034C, HHSN261201000035C, HHSN261201000029C, HHSN261201000031C, HHSN261201000028C, and HHSN261201000030C. The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. This article was produced by employees of the US government as part of their official duties and, as such, is in the public domain in the United States of America. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enewold, L., Geiger, A.M., Zujewski, J. et al. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 151, 149–156 (2015). https://doi.org/10.1007/s10549-015-3366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3366-7