Abstract
Overall survival (OS) has been debated as the most important clinical endpoint in metastatic breast cancer (MBC) trials mainly because survival could be influenced by treatment after progression in an era of effective subsequent-line agents. We conducted a search strategy using PubMed for all the phase 3 trials in the last two decades evaluating survival outcome in MBC. We investigated the frequency of trials reporting survival outcome and response/resistance to treatment beyond progression. One hundred fifteen trials met our eligibility criteria: 69 (60 %) evaluated chemotherapy regimens (group A), 32 (28 %) evaluated targeted therapies (group B), and 14 (12 %) focused on endocrine treatment (group C). An OS benefit was demonstrated in approximately 22 % of the trials in each group. Less than 10 % of the trials in group A and B reported response data after progression on trial therapy. Post-progression treatment resistance was only reported in group A in 3 % (2/69) of the trials. In addition, the number of lines of treatment used post-progression was reported in 14 % (10/69), 9.4 % (3/32), and 14 % (2/14) of the trials in group A, B, and C, respectively. Post-progression survival and its effect on OS was reported in only 1 % (1/69), 3 % (1/32), and 7 % (1/14) of the trials for group A, B, and C respectively. A clear paucity of post-progression treatment information is noted in the majority of the phase 3 trials for MBC. We do know that OS can be affected partially or directly by treatments used after progression. In order to assess the true clinical benefit of a new drug and to have a complete evaluation of OS outcome, a detailed collection of post-progression treatment information is required and should be mandated in MBC trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women worldwide, with nearly 1.7 million new cases diagnosed in 2012 [1]. It is also the most common cause of cancer death among women (522,000 deaths in 2012) [1]. Its incidence has been increasing in most regions of the world; however, mortality rates are decreasing as a result of increased and better screening methodologies, as well as more effective and potent treatments (e.g., improved adjuvant therapies) [2]. Furthermore, nowadays, most of the patients diagnosed with metastatic breast cancer (MBC) receive and benefit from several lines of treatments that contribute to their prolonged survival [3, 4].
Overall survival (OS) has been historically considered as the “gold standard” and most objective endpoint used in phase 3 trials in cancer diseases in general and specifically in MBC [5]. Nevertheless, OS has been debated as the most important clinical endpoint. OS is affected by treatments used after progression on the trial drug, especially when such therapies are effective and given that there are number of lines of treatment available for patients with MBC. In addition, in order to detect statistically significant OS differences, studies have to be well powered, include large number of patients, and have a long follow-up which is becoming more challenging in an era where treatments’ standards are changing rapidly [6].
On another hand, progression-free survival (PFS) is being used more frequently as a primary endpoint in phase 3 trials [7] as it is less affected by post-progression treatments’ effectiveness and other causes of death. PFS events occur more quickly and frequently than OS events, and fewer patients are required to obtain such data. Despite the fact that some studies found a positive correlation between PFS and OS [8], its validation as a surrogate endpoint for OS is still controversial in BC [9, 10].
We do recognize that current therapies in MBC represent a small numerical benefit, and more work is needed to improve the outcome for MBC; hence, it seems important to know what happens after the first progression.
At the end, the main goal is to extend patients’ survival while maintaining or improving patient’s quality of life. In this setting, we analyzed the recent literature trying to answer some specific questions: (1) Do MBC trials capture post-progression survival data? (2) Do MBC trials capture information on treatments received post-progression? (3) Is there a difference in trials being conducted for chemotherapeutic, targeted, and endocrine agents?
Methods
Search technique
We conducted a manual and electronic web-based search using MEDLINE/PubMed to review all phase 3 trials conducted in MBC and published in English in the last two decades (1994–2014). We used combinations of these phrases or keywords: “metastatic breast cancer,” “advanced breast cancer,” and “phase 3 trial and breast cancer.” We reviewed all randomized phase 3 trials for MBC addressing the survival outcome as a primary or secondary endpoint. Survival outcome was assessed with overall survival (OS) and either with progression-free survival (PFS), event-free survival (EFS), time to progression (TTP), or time to treatment failure (TTF). In order to simplify the analysis, we will use PFS when referring to PFS, EFS, TTP, and TTF. We retained all studies comparing chemotherapy treatments, endocrine treatment, and/or targeted therapies excluding trials for supportive care including those evaluating bone targeted agents (e.g., bisphosphonates, RANKL inhibitors, etc.) radiotherapy and vaccines.
Endpoints
Primary endpoint was to assess the frequency of trials that (1) report post-progression survival (PPS) defined as median OS minus median PFS and discuss its effect on OS, (2) discuss treatment efficacy (response rate, clinical benefit, etc.) and treatment resistance (primary or secondary) after progression on trial drugs, and (3) discuss and report the number and type of treatments used after progression. Secondary endpoint was to report the frequency of OS benefit and PFS benefit (statistically significant) in all phase 3 trials in the 1st line setting and beyond the first line of treatment. First-line treatment was defined as any treatment given (chemotherapy or endocrine therapy or targeted therapy or any combination of those) for MBC patients who were systemic-treatment naïve in the metastatic setting.
Results
Trials identification
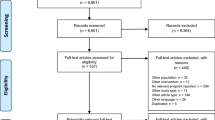
We identified 1660 manuscripts when searching for the sentences: “phase 3 trial” and “metastatic breast cancer” and 1188 articles when we searched for the following sentences: “phase 3 trial” and “advanced breast cancer (ABC).” We retained and analyzed 115 papers that met the search and eligibility criteria: phase 3 randomized trial in MBC (any line of treatment) comparing two or more arms of treatment (chemotherapy, endocrine treatment, and/or targeted therapies). None of the selected manuscripts re-reported any other included study, and any multiple reports had the same results. Sixty-nine trials (60 %) were comparing chemotherapy treatments defined as group A, 32 trials (28 %) had targeted therapies at least in one arm, defined as group B, and 14 trials (12 %) compared endocrine treatments defined as group C.
Trials endpoints and lines of treatment
Overall, 68 trials (59 %) evaluated treatments in the 1st line setting and 47 trials (41 %) evaluated treatments beyond the 1st line. The details of line of treatments by group are presented in Table 1.
OS was a primary and a secondary endpoint in 14 trials (12 %) and 96 trials (83 %), respectively. PFS was a primary and a secondary endpoint in 74 trials (64 %) and 54 trials (47 %), respectively. Some trials used PFS as a primary endpoint and TTP as a secondary endpoint; hence, the previous percentages reported. The detailed endpoints by group are described in Table 2.
Survival benefit
A statistically significant OS benefit was reached in 25 trials (22 %). Among those, 14 trials (12 %) were evaluating treatments in the 1st line setting and 11 trials (10 %) were beyond the 1st line. A statistically significant PFS benefit was reached in 65 trials (57 %). Thirty-nine trials were comparing treatments in the 1st line setting (34 %) and 26 trials were comparing treatments beyond the 1st line setting (23 %). Survival benefit data by group are detailed in Table 3.
Post-progression data
All over, a paucity of trials reported and discussed PPS data and its effect on the OS. Most of the trials did not detail any information post-progression, once the endpoint was reached. Ten trials reported response data post-progression (response rates, PFS after progression) (9 %). Only 3 trials reported PPS data and analyzed its effect on OS (3 %). In addition, 2 trials discussed treatment resistance on the next line of therapy after progression on trial drug (2 %). Furthermore, the number of lines of treatment post-progression was detailed in only 15 trials (13 %). As for the type of treatments used post-progression, it was reported and discussed in approximately third of the trials [41 trials (36 %)]. The details of PPS data reported in the trials by group are presented in Table 4.
Discussion
Approximately 5–10 % of BC cases are metastatic at diagnosis, and up to 30 % of node negative and 70 % of node positive BC cases subsequently develop metastases [11]. Significant achievements and improvement in outcomes were made in the last decade in the treatment of MBC patients [12, 13]. In a population-based analysis of survival outcomes in MBC, the introduction of new agents over the past decade, such as the taxanes, aromatase inhibitors, and trastuzumab, was associated with a significant improvement in OS across the population [3]. The most significant OS improvement was for the Her2 positive population with the addition of herceptin [14–17]. Going further, the updated results from the CLEOPATRA trial showed after a median follow-up of 50 months, a persistent OS benefit with a median survival of 56.5 months in the study arm (HR = 0.68, p = 0.0002) [18]. However, the benefit of treatments used in MBC is still in general small even if statistically significant, and more efforts are needed to improve outcomes in that setting. The prognosis remains poor and therapeutic goals are palliative in nature [19]. Moreover, the cytotoxic chemotherapy options for second and subsequent-line of treatment have expanded in the last decade and improved the disease outcome despite a general consensus that the benefit in that setting is uniformly poor [19–21].
It is noteworthy to mention that most of the phase 3 trials conducted in MBC are still evaluating chemotherapy regimens. After reviewing the literature, we found that 60 % of the trials used a chemotherapy regimen at least in one arm, one-third of the trials used targeted therapies, and only 12 % evaluated endocrine treatments that are usually less toxic and more tolerated by patients. This clearly shows that efforts are still being made to improve survival outcome using the most aggressive and potent therapies in the trials conducted despite the prolonged survival and the relative good prognosis particularly for the hormone receptor positive BC cases with an indolent nature [22]. In parallel, the majority of the phase 3 trials published compared treatments in the 1st line (59 %) and this was consistent and did not differ by group (A, B and C), showing that most of the new treatments and trials are developed in the 1st line setting in an attempt to control the disease at diagnosis as early as possible.
On another hand, despite the fact that OS is still the “gold standard” endpoint in MBC trials, less than 15 % of the trials were designed with OS as a primary endpoint and the majority of the trials had OS as their secondary endpoints. On the contrary, PFS was more frequently used as a primary endpoint (approximately 64 %) and this was also consistent among all groups. This shows the complexity and the challenge of designing trials with enough power to have an OS benefit in their primary endpoint and the current trend to use PFS as a primary endpoint in most of the trials.
Twenty-two percent of the phase 3 trials showed a significant OS benefit which is in line with another review [23] and marginally higher than other previous reports [24, 25]. Fifty-seven percent of the trials reached significance for PFS. Choosing the right endpoint for phase 3 trials for MBC remains a challenge with the new advances and therapies.
If a new drug is truly efficient, then theoretically and ideally it should not be affected by post-progression treatments and the benefit should persist independently. However, there are concerns that the efficacy of drugs measured by OS may be diluted in clinical trials, thereby underestimating their true clinical benefit. In addition, the inherent natural history of the specific tumor has also an impact on OS. That concern is based on the assumption that subsequent lines of therapy are more effective in the control arm than in the treatment arm, or that the biology of the treatment arm has changed because of exposure to the study drug; however, this is not supported by evidence [26]. Post-progression treatments that work better in the standard arm than the experimental arm will lead to a smaller OS difference. If they are equally effective, then the absolute OS benefit is not affected but the relative improvement in OS would be smaller. Moreover, when patients cross over to the experimental treatment, a smaller OS difference could be observed if any [27]. An example to our statement would be the TAnDEM study where the addition of trastuzumab to letrozole showed only an improvement in PFS and not OS; however, 70 % of the patients on the letrozole arm alone crossed over [28]. A second example is the addition of ixapebilone to capecitabine that also did not show an improvement in OS despite a benefit in PFS; however, in this situation, there were no crossing over, so either truly the combination is not better or because the patients received active therapies after progression that the effect of the experimental drug was blunted [29]. Another interesting example would be the EGF100151 trial where overall, the addition of lapatinib to capecitabine did not improve OS; however, when the patients who crossed over to this combination were removed, a significant OS was reported [30]. Still we have to mention that this was not in the primary intent-to-treat analysis. The EMBRACE trial is an example of a persistent OS benefit; several explanations were given in that setting, such as no efficient post-progression treatments were available after the 4th line and the lack of cross over [31]. The same drug (Eribulin) was tested in an earlier setting compared to capecitabine, and no significant benefit was shown in the whole population [32]. Based on a recent model [33], OS would be an appropriate primary endpoint when median PPS is short (less than 12 months); this involves patients who did not benefit significantly from post-progression treatments, and PFS would be a better primary endpoint when median PPS is longer than 12 months where the patients did benefit significantly from post-progression therapies. This means that data after progression might be important for us to assess the whole picture and confirm the efficacy of a new treatment or the opposite.
The PPS data and the analysis of its effect on OS were reported in few studies only. Going further, only a small number of trials analyzed patient outcomes and treatment after progression on the study treatment. The picture is unclear in most of the phase 3 trials published on what happens after the first progression and OS might be an incomplete endpoint in that setting. Few trials reported the efficacy of treatments beyond progression and reported the response rates and PFS after the first progression. In the group A and B, less than 10 % of the trials reported such data, while 21 % of the trials in the group C provided this information. Indeed, a higher percentage is noticed in the group C, but because of the small number of trials in that group, we have to be careful in analyzing the results and cautious in our conclusions (only 14 trials in group C). Out of 115 trials analyzed, we found only 3 trials, 1 in each group outlining the outcome after progression and how survival after progression defined as PPS affects the OS. Despite its importance, treatment resistance beyond treatment trial was barely mentioned and discussed. Only 2 trials in the group A reported such data. It is crucial to know if a response was achieved after progression when patients were treated, then a resistance developed and patients progressed or if the treatment post progression was ineffective from the beginning and patients were considered to have a primary resistance. The number of lines of treatments used post-progression was reported in approximately 10 % of the trials in each group but without complete details on every treatment or its efficacy. On another hand, the type of treatments used beyond progression was well reported in all groups with more than 25 % of the trials at least mentioning and citing the type of treatments post-progression either it was chemotherapy, endocrine treatment, or targeted therapies.
At the end, we need to have the complete picture during the journey of a patient starting from the beginning of their treatment till death. Once a primary endpoint is reached, post-progression data might be also important in order to evaluate the real clinical benefit of a new treatment and make the right decisions concerning drugs approval. A good example is Bolero 2 trial where there was no statistical OS benefit from adding everolimus to exemestane after a median follow-up of 39 months; however, a significant statistical persistent PFS difference was reported (7.8 vs. 3.2 months; HR = 0.45 [95 % CI 0.38–0.54]; p < 0.0001) [34]. The PPS was similar in both arms, and the trial had PFS as a primary endpoint and OS as a secondary endpoint. Whether to consider the combination of exemestane and everolimus a new standard is still a matter of debate; the true clinical benefit is questionable with the rate of toxicity reported with this regimen (55 % of grade 3/4 adverse events and 33 % of serious adverse events) and the lack of OS benefit [35]. Collecting the complete PPS information can help guide the clinician to truly assess the benefit of integrating a new therapy in the metastatic treatment and also inform patient and clinicians on likelihood of effectiveness of other available treatments post-progression.
OS remains the “gold standard” and most objective endpoint to use in phase 3 trials, and PFS is becoming the most frequently used primary endpoint in randomized MBC studies. Both are essential to evaluate; however, to assess the true efficacy of a new treatment, we need to know the complete treatment information that includes post-progression treatment. Ideally, we should mandate to report in every published phase 3 trial such data; this could be done if OS is the primary endpoint. However, this might lead to a delay in the approval of “truly” efficient drugs if we have to wait for OS results. Another way would be mandating the usage of 2 primary endpoints, where one would be OS. Yet, the best practical way to capture this data is to be determined.
Conclusion
In conclusion, our review notes that only few phase 3 trials in MBC provide data on post-progression survival, treatment, and correlate such information with OS. Detailed post-progression treatment information is needed in MBC clinical trials for a complete evaluation of OS and a true assessment of the efficacy of new drugs and their clinical benefit.
References
Ferlay J, Soerjomataram I, Ervik M et al. (2013) GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. Available from http://globocan.iarc.fr. Accessed 13 Dec 2013
Canadian Cancer Society’s Advisory Committee on Cancer Statistics (2013) Canadian cancer statistics 2013. Canadian Cancer Society, Toronto
Chia SK, Speers CH, D’yachkova Y et al (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979
Gennari A, Conte P, Rosso R et al (2005) Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 104(8):1742–1750
Smith I (2006) Goals of treatment for patients with metastatic breast cancer. Semin Oncol 33(1 Suppl 2):S2–S5
Di Leo A, Bleiberg H, Buyse M (2003) Overall survival is not a realistic end point for clinical trials of new drugs in advanced solid tumors: a critical assessment based on recently reported phase III trials in colorectal and breast cancer. J Clin Oncol 21:2045–2047
Saad ED, Katz A (2009) Progression-free survival and time to progression as primary end points in advanced breast cancer: often used, sometimes loosely defined. Ann Oncol 20:460–464
Sherrill B, Hirst C, Wu Y et al (2007) Correlation between time to progression and overall survival in patients with metastatic breast cancer. In: 12th annual international meeting of the International Society for Pharmacoeconomics and Outcomes Research, 19–23 May 2007
Burzykowski T, Buyse M, Piccart-Gebhart MJ et al (2008) Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol 26:1987–1992
Miksad RA, Zietemann V, Gothe R et al (2008) Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care 24:371–383
Cardoso F, Harbeck N, Fallowfield L (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis treatment and follow-up. Ann Oncol 23(Suppl 7):vii11–vii19
Andre F, Slimane K, Bachelot T et al (2004) Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 22(16):3302–3308
Mauri D, Polyzos NP, Salanti G et al (2008) Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst 100(24):1780–1791
Harris CA, Ward RL, Dobbins TA et al (2011) The efficacy of HER2-targeted therapy agents in metastatic breast cancer: a meta-analysis. Ann Oncol 22:1308–1317
Johnston SR (2011) The role of chemotherapy and targeted agents in patients with metastatic breast cancer. Eur J Cancer 47:38–47
Dean-Colomb W, Esteva FJ (2008) Her2-positive breast cancer: herceptin and beyond. Eur J Cancer 44:2806–2812
Kawalec P, Lopuch S, Mikrut A (2014) Effectiveness of targeted therapy in patients with previously untreated metastatic breast cancer: a systematic review and meta-analysis. Clin Breast Cancer pii:S1526-8209(14)00226-2 [Epub ahead of print]
Swain SM, Baselga J, Kim SB et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734
Jones SE (2008) Metastatic breast cancer: the treatment challenge. Clin Breast Cancer 8(3):224–233
Roché H, Vahdat LT (2011) Treatment of metastatic breast cancer: second line and beyond. Ann Oncol 22(5):1000–1010
Cardoso F, Di LA, Lohrisch C et al (2002) Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann Oncol 13(2):197–207
Zhang XH, Giuliano M, Trivedi MV et al (2013) Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 19(23):6389–6397
Saad ED, Katz A, Buyse M (2010) Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol 28(11):1958–1962
Verma S, McLeod D, Batist G et al (2011) In the end what matters most? A review of clinical endpoints in advanced breast cancer. Oncologist 16(1):25–35
Wilcken N, Dear R (2008) Chemotherapy in metastatic breast cancer: a summary of all randomized trials reported 2000–2007. Eur J Cancer 44:2218–2225
Cheema PK, Burkes RL (2013) Overall survival should be the primary endpoint in clinical trials for advanced non-small-cell lung cancer. Curr Oncol 20(2):e150–e160
Korn EL, Freidlin B, Abrams JS (2011) Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol 29(17):2439–2442
Kaufman B, Mackey JR, Clemens MR et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM Study. J Clin Oncol 27:5529–5537
Sparano JA, Vrdoljak E, Rixe O (2010) Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 28:3256–3263
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Cortes J, O’Shaughnessy J, Loesch D et al (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923
Kaufman PA, Awada A, Twelves C et al (2015) Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 33(6):594–601
Broglio KR, Berry DA (2009) Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 101:1642–1649
Piccart M, Hortobagyi GN, Campone M et al (2014) Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 25(12):2357–2362
Tannock IF, Pond GR (2014) Everolimus, when combined with exemestane, adds toxicity with minimal benefit for women with breast cancer. Ann Oncol 25(10):2096. doi:10.1093/annonc/mdu371
Conflict of interest
S. Verma: Advisory Board—Roche, EISAI, Amgen, Novartis, Astra Zeneca. All remaining authors have declared no conflicts of interest.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raphael, J., Verma, S. Overall survival (OS) endpoint: an incomplete evaluation of metastatic breast cancer (MBC) treatment outcome. Breast Cancer Res Treat 150, 473–478 (2015). https://doi.org/10.1007/s10549-015-3342-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3342-2