Abstract
It has been observed that women living in urban areas have a higher mammographic density (MD) compared to women living in rural areas. This association might be explained by regional differences in reproductive and lifestyle factors or perhaps by variation in exposure to ambient air pollution as air pollution particles have been described to show estrogenic activity. We investigated the association between degree of urbanization and MD, and aimed to unravel the underlying etiology. 2,543 EPIC-NL participants were studied, and general linear models were used. Urbanization was categorized into five categories according to the number of addresses/km2. Information on reproductive and lifestyle factors was obtained from the recruitment questionnaire. Air pollution exposure was estimated using land-use regression models. MD was expressed as percent density (PD) and dense area (DA), and was quantified using Cumulus. Women living in extremely urbanized areas had a higher PD (21.4 %, 95 % confidence interval (CI) 20.5–22.3 %) compared to women living in not urbanized areas (16.1, 95 % CI 14.5–17.8 %, P trend < 0.01).The association persisted after adjustment for reproductive and lifestyle factors as well as for individual exposure to air pollution (adjusted PDextremely_urbanized = 22.1 %, 95 % CI 18.0–26.5 % versus adjusted PDnot_urbanized = 16.9 %, 95 % CI 13.0–21.2, P trend < 0.01).The results for DA showed close similarity to the results for PD. We found evidence that degree of urbanization is associated with MD. The association could not be explained by differences in reproductive and lifestyle factors or by variation in air pollution exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been observed that women living in urban areas have a higher mammographic density (MD) compared to women living in rural areas [1, 2]. Perry et al. investigated the association by comparing MD values of women living in London with MD values of women living in rural areas around London [1]. Viel et al. investigated this association within the French national breast cancer screening program [2]. Both studies observed more women in the highest MD category in urban than in rural areas. The underlying cause could not be elucidated, but MD risk factors might have been responsible, because the two studies had only limited data available on these factors (i.e., age, hormone replacement therapy (HRT) use, and marital status).
MD is a well-known breast cancer risk factor [3] and is thought to represent the estrogen exposure cumulative during life [4]. Consequently, MD is associated with reproductive factors, such as parity, menopausal status, and HRT use [5–8]. In addition, associations with lifestyle factors like smoking habits and alcohol consumption have been observed [6, 7, 9]. Therefore, it might be that observed associations between degree of urbanization and MD are—at least partly—explained by variation in reproductive and lifestyle factors. On the other hand, it is hypothesized that degree of urbanization represents differences in exposure to ambient air pollution [1, 2]. Air pollution particles could possibly affect MD via the estrogen-related pathway as Clemons et al. have demonstrated that air pollution particles have estrogenic activity [10].
The aim of this study was to investigate the association between degree of urbanization and MD within the Dutch screening population. Since we have information on many MD risk factors, we could additionally examine to what extent this relationship is explained by variation in well-known MD risk factors. Furthermore, we examined whether variation in air pollution exposure could explain the relationship under study.
Methods
Study population
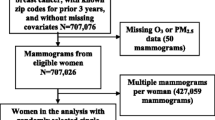
The study was performed within the EPIC-NL study [11], which is a Dutch cohort study that participates in the European Prospective Investigation into Cancer and Nutrition (EPIC) study [12, 13]. EPIC is a European-wide cohort study designed to investigate the association between nutrition and cancer. For this study, we used data from a subcohort of EPIC-NL, which is the Prospect cohort [14]. Prospect-EPIC participants were recruited through the breast cancer screening program in Utrecht and vicinity. This regional program is part of the national Dutch cancer screening program. From 1993 to 1997, 17,357 individuals aged 49–69 years were included when visiting the screening program. The design of both EPIC and Prospect-EPIC has been described in detail elsewhere [12, 14].
MD was not a standard measurement in the screening program and therefore assessed for two subpopulations (together n = 2,588) [15, 16]. The first subpopulation comprised controls of a nested case-control study on age-related changes in MD (n = 1,156) [15].This study included women without a history of (breast) cancer, except non-melanoma skin cancer, and with at least 3 mammographic examinations available. The second population comprised women of a 10 % random sample of the total Prospect-EPIC cohort (n = 1,432) [16].
Women with missing postal code values were excluded (n = 45), leaving 2,543 women for the analyses. All participants gave written informed consent. The study was approved by the Institutional Review board of the University Medical Center Utrecht.
Assessment of degree of urbanization
Data on degree of urbanization were derived from Statistics Netherlands [17]. Since recruitment was between 1993 and 1997 with a mean of 1995, we obtained data from 1995. Urbanization at a participant address was defined as the number of addresses per km2 for every municipality and was categorized according to the classification from Statistics Netherlands: not urbanized (0–499 addresses/km2), hardly urbanized (500–999 addresses/km2), moderately urbanized (1,000–1,499 addresses/km2), strongly urbanized (1,500–2,499 addresses/km2), and extremely urbanized (>2,499 addresses/km2) [18].
Assessment of reproductive and lifestyle characteristics
Information on reproductive and lifestyle characteristics was obtained from the recruitment questionnaire and described in more detail under Statistical analysis. Neighborhood socio-economic status (SES) was defined as the percentage high-income households (i.e., at or above the national 80th percentile) per postal code. These data were derived from Statistics Netherlands and were only available for the year 2001 [17].
Assessment of air pollution exposure
Air pollution exposure was estimated using land-use regression models from the European Study of Cohorts for Air Pollution Effects (ESCAPE) [19, 20]. This technique has been described in detail elsewhere [19, 20]. In brief, nitrogen oxides (NO x ), nitrogen dioxide (NO2), and particulate matter with an aerodynamic diameter of less than 10 μm (PM10) as well as less than 2.5 μm (PM2.5), and blackness of the PM2.5 exposed filter (PM2.5 abs) were measured during different seasons at several representative measurement sites in the Netherlands and Belgium (n = 80 NO x and NO2 sites; n = 40 PM sites) between February 2009 and February 2010 [21, 22]. The amount of particulate matter with an aerodynamic diameter between 2.5 and 10 μm (PMcoarse) was calculated by subtracting PM10 from PM2.5. For each air pollutant component, a land-use regression model was developed. The mean annual concentration of the air pollutant component was used as the dependent variable, and predictor variables collected using geographic information systems for each monitoring site were used as independent variables. These predictor variables were related to traffic intensity and extensiveness of road network, as well as land use and population density. The home address of each Prospect-EPIC participant (i.e., postal code and number) at recruitment was recoded into standard Dutch geographic coordinates. The air pollution exposure for each geographic coordinate in the Prospect-EPIC cohort was estimated using land-use regression models.
Assessment of MD
The film-screen mammograms were digitized with a Canon CFS300 scanner. The left mediolateral oblique view was used for density assessment. MD was semi-automatically quantified using Cumulus software (University of Toronto, Canada). For each image, the pectoral muscle was manually masked out. Subsequently, the reader set a threshold to outline the breast area (BA). A second threshold distinguished the dense area (DA) from the non-dense area. The software program then determined the number of pixels within the DA as well as within the total BA. Percent density (PD) was calculated by dividing the DA by the total BA multiplied by 100. For both studies, one reader assessed all mammograms [16, 23]. The intraclass correlation coefficients for the between-batch correlation for PD, DA, and BA were, respectively, 0.96, 0.96, and 1.00 for the study by Lokate et al. [23] and 0.91, 0.93, and 1.00 for the 10 % random sample [16].
Statistical analysis
To illustrate the MD distribution across degree of urbanization levels, continuous PD measurements were categorized. Categories were defined as to resemble the distribution of the often used clinical American College of Radiology Breast Imaging-Reporting and Data System (ACR BI-RADS) density categories: 1 almost entirely fatty, 2 scattered fibroglandular densities, 3 heterogeneously dense, and 4 extremely dense [24]. The distribution of the Breast Cancer Surveillance Consortium data described by Kerlikowske was used (i.e., ACR BI-RADS density distribution: 1 = 9.5 %; ACR 2 = 47.5 %; ACR 3 = 37.3 %; ACR 4 = 5.7 %) [25].
For the analysis of covariance, MD values (i.e., PD and DA values) were transformed to make the distributions normal via the equation: \(X' = \sqrt {\left( {x + 0.5} \right)}\) [26]. Degree of urbanization and categorical confounders were included as fixed factors and continuous confounders as covariates. Model 1 was adjusted for subpopulation (population 1, population 2), age (continuous, years), and body mass index (BMI) (continuous, kg/m2). To determine to what extent the association was explained by variation in reproductive and lifestyle factors, we additionally adjusted for the following factors (model 2): age at menarche (<12, 12–14, >14 years), oral contraceptive (OAC) use (never, past, current), age at first delivery (nulliparous, ≤25, >25 years), number of children (continuous), breast feeding (never, ever), menopausal status (premenopausal, perimenopausal, postmenopausal ≤50 years, postmenopausal >50 years), HRT use (never, past, current), physical activity (inactive, moderately inactive, moderately active, active), smoking (never, past, current), alcohol user (yes, no), alcohol use (continuous, g/day), educational level (primary school, technical/professional school, secondary school, university degree), and percentage high-income households at neighborhood level (continuous). Women were considered premenopausal when they had had at least 6 menstrual periods in the 12 months prior to inclusion and were not currently using OAC or HRT. Women without any menstrual periods during the last 12 months either natural or surgical were categorized as postmenopausal. The perimenopausal category included women who reported having had less than 6 menstrual cycles during the last 12 months or women who used OAC or HRT at the time of inclusion or when they had equivocal data. In model 3, we additionally adjusted for environmental factors (i.e., NO2, NO x , PM2.5, PM2.5 abs, PM10, and PMcoarse). The effect of each air pollutant on the association under study was investigated as well as the combined effect of all air pollutants together.
As the association between air pollution exposure and MD has not been studied yet, we additionally assessed this relationship using linear regression analysis. Each air pollutant under study was analyzed per interquartile (IQR) increase in exposure.
Sensitivity analyses were performed to assess robustness of the association under study. First, results were stratified by menopausal status at recruitment as estrogen levels decrease after menopause, and it has been hypothesized that an estrogen-related pathway might be involved in the association under study [10, 27]. Since smoking behavior is expected to be related to degree of urbanization [17] and results are prone to residual confounding due to an imprecise measurement of exposure to cigarette smoke, we repeated the analyses in never smokers (n = 1,087). Lastly, results were stratified by subpopulation in order to determine whether both datasets showed similar results.
The back transformed means and confidence intervals (CI) of density measures were calculated via the equation: \(X' = \sqrt {\left( {x + 0.5} \right)}\) [26].
Two-tailed p values of <0.05 were considered as statistically significant. The statistical analyses were performed using IBM SPSS Statistics version 20.
Results
In total, 2,543 women were included with a median age at recruitment of 57 years (IQR = 52–62 years). Of these women, 1,929 (75.9 %) were postmenopausal. The median PD was 20.7 % (IQR = 11.6–32.5 %) and the median DA was 25.0 cm2 (IQR = 15.4–37.2 cm2).
Table 1 shows the characteristics of the study population at recruitment for the upper and lower level of degree of urbanization. Women living in extremely urbanized areas were more frequently current OAC users and more often nulliparous compared to women living in not urbanized areas. In addition, they were more frequently current smokers and lived in lower SES neighborhoods. The median air pollution concentrations were higher in extremely urbanized areas compared to not urbanized areas, especially NO2 and NO x concentrations.
Figure 1 presents the results for PD values categorized according to BI-RADS density-like categories. Women living in extremely urbanized areas were more frequently categorized into category 3 (heterogeneously dense) or 4 (extremely dense) (together 48.2 %) than women living in not urbanized areas (28.2 %).
Table 2 shows the association between degree of urbanization and mean PD and mean DA. Women living in extremely urbanized areas had a higher mean PD (21.4 %, 95 % CI 20.5–22.3 %) than women living in not urbanized areas (16.1 %, 95 % CI 14.5–17.8 %). Although the P trend was significant (P trend < 0.01), no clear dose–response relationship was found. The association persisted after adjustment for other well-known MD risk factors as well as for individual exposure to air pollution (adjusted PDextremely_urbanized = 22.1 %, 95 % CI: 18.0–26.5 % versus adjusted PDnot_urbanized = 16.9 % (95 % CI 13.0–21.2), P trend < 0.01). The results for mean DA showed close similarity to the results for PD.
In Table 3, we also showed the main effects of each air pollutant. High exposure to NO2 was statistically significantly associated with high MD and this association was explained by variation in degree of urbanization. High exposure to PM2.5 abs and PM10 were borderline associated with high MD. After adjustment, these relationships could no longer be observed.
To assess the robustness of the results, three sensitivity analyses were performed. Firstly, we stratified the results by menopausal status. Although the number of premenopausal women is too low to test for heterogeneity (n = 251), the results suggest the associations appear to be more clearly present in postmenopausal women (data not shown). In the second sensitivity analysis, results were stratified by subpopulation, and in the third, the analyses were repeated in never smokers. Although the smaller sample size resulted in larger CIs, the results of both sensitivity analyses did not alter our conclusions (data not shown).
Discussion
The present study provides evidence that women living in extremely urbanized areas have a higher mean PD than women living in not urbanized areas. The association could not be explained by differences in MD risk factors or by variation in air pollution exposure. Although the P trend was significant, no clear dose–response relationship was found.
Up to now, three studies have been conducted on the association between urbanization and MD [1, 2, 28], and in two of these a similar urbanization gradient in MD has been observed [1, 2]. In line with our results, Perry et al. observed more women in the highest MD category in London compared to rural areas around London (odds ratio (OR)urban_versus_rural = 1.32, 95 % CI 1.04–1.70) [1]. A study conducted within the French national breast cancer screening program found a weaker association, but still in the same direction (ORurban_versus_rural = 1.07, 95 % CI 1.03–1.12) [2]. Aitken et al. performed a study within the UK Age trial, which is a breast cancer screening trial for women aged 40–48 years [28]. No association between urbanization and MD was found, which might have been caused by the limited variation in urbanization gradient [28].
Although we adjusted for many well-known MD risk factors as well as for exposure to air pollution, we were not able to elucidate the underlying mechanism. We cannot exclude that we underestimated the effect of air pollution in this pathway, due to misclassification of air pollution exposure. Misclassification might have been caused by the fact that exposure to air pollution was based on 2009–2010 measurements, whereas MD was assessed on mammograms which were taken on average 15 years earlier, 1993–1997. However, we expect this misclassification to be limited as it has been observed that, although the absolute air pollution concentrations have changed over time, the spatial air pollution contrasts remain rather stable [29–32]. In the Netherlands, a small decreasing trend in NO2 concentrations has been observed between 1999 and 2007 [33]. Nevertheless, Eeftens et al. found evidence that NO2 measurements conducted in 2007 showed high agreement with NO2 measurements taken in 1999–2000 (i.e., absolute concentrations, R 2 = 0.86) [32]. In addition, the land-use regression model that was developed using measurements from the year 2007 (i.e., NO2 concentrations and predictor variables) was able to predict NO2 measurements for the years 1999–2000 well (R 2 = 0.77).
The limited influence of air pollution exposure on the association under study might also be explained by the fact that other environmental factors, besides air pollutants, play a more important role. For example, organochlorine pollutants (i.e., industrial chemicals) are described to have estrogenic properties and organochlorine concentrations have been observed to be higher in urban than in rural areas [34–36]. However, one should note that no conclusive evidence has been found for an association between organochlorine concentrations and breast cancer risk [37]. Unfortunately, we could not study the influence of organochlorine or other persistent organic pollutants, because these data were not available.
The present study has some strengths and limitations that need to be addressed. A possible limitation of the study is the small study area (2,400 km2) and thus limited variation in degree of urbanization. Although the variation within the region studied, or within the Netherlands, may be limited compared to other regions in the world, it still allowed us to observe an urbanization gradient in MD. In a region with larger variation, one would expect to find an even stronger association. Another possible limitation is that we assessed lifestyle habits only at recruitment, whereas it might be that lifestyle factors have lifetime cumulative effects or influence MD mainly in early life. Furthermore, air pollution exposure was estimated for the home address at recruitment, but we do not know how long a participant had already lived at her recruitment address. This might have caused misclassification of lifestyle factors and air pollution exposure leading to residual confounding. One of the major strengths of our study is the availability of extensive information on MD risk factors, including data on air pollution exposure. Another strength of the study is its size. With 2,543 participants, our study is one of the largest studies conducted so far on this topic.
Despite the fact that we measured many MD risk factors, any difference in those could not explain the association between degree of urbanization and MD. Nevertheless, from a national health perspective, it is crucial to be aware of regional variation in MD whatever the cause of this variation may be. MD is an important breast cancer risk factor [3]. We observed a mean PD difference between extremely urbanized and not urbanized areas of 5 %. A study by Boyd et al. showed that every 1 % increase in density, leads to a 2 % increase in the relative risk to develop breast cancer, which translates to a 10 % increase in relative risk of breast cancer in case of an increase of 5 % in PD [38]. The direct relationship between degree of urbanization and breast cancer risk has already been studied and some studies observed a higher breast cancer incidence in urban areas compared to rural areas [39, 40].Besides this, high MD strongly decreases mammographic tumor detectability, which leads to more missed cases [25, 41]. This dual effect of MD on cancer incidence and sensitivity of mammography, results in lower breast cancer screening performance in these women compared to women with fatty breasts.
In conclusion, the results of this study showed that women living in extremely urbanized areas had a higher MD than women living in not urbanized areas which could not be explained by other MD risk factors or by differences in air pollution exposure.
Abbreviations
- ACR BI-RADS:
-
American College of Radiology Breast Imaging-Reporting and Data System
- BA:
-
Breast area
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DA:
-
Dense area
- EPIC:
-
European Prospective Investigation into Cancer and Nutrition
- ESCAPE:
-
European Study of Cohorts for Air Pollution Effects
- HRT:
-
Hormone replacement therapy
- IQR:
-
Interquartile range
- MD:
-
Mammographic density
- NO x :
-
Nitrogen oxides
- NO2 :
-
Nitrogen dioxide
- OAC:
-
Oral contraceptive
- OR:
-
Odds ratio
- PD:
-
Percent density
- PM2.5 :
-
Particulate matter with an aerodynamic diameter of less than 2.5 μm
- PM2.5 abs :
-
Blackness of the PM2.5 exposed filter
- PMcourse :
-
Particulate matter with an aerodynamic diameter between 2.5 and 10 μm
- PM10 :
-
Particulate matter with an aerodynamic diameter of less than 10 μm
- SES:
-
Socio-economic status
References
Perry NM, Allgood PC, Milner SE, Mokbel K, Duffy SW (2008) Mammographic breast density by area of residence: possible evidence of higher density in urban areas. Curr Med Res Opin 24(2):365–368
Viel JF, Rymzhanova R (2012) Mammographic density and urbanization: a population-based screening study. J Med Screen 19(1):20–25
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15(6):1159–1169
Clemons M, Goss P (2001) Estrogen and the risk of breast cancer. N Engl J Med 344(4):276–285
El-Bastawissi AY, White E, Mandelson MT, Taplin SH (2000) Reproductive and hormonal factors associated with mammographic breast density by age (United States). Cancer Causes Control 11(10):955–963
Stone J, Warren RM, Pinney E, Warwick J, Cuzick J (2009) Determinants of percentage and area measures of mammographic density. Am J Epidemiol 170(12):1571–1578
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 11(7):653–662
Butler LM, Gold EB, Greendale GA, Crandall CJ, Modugno F, Oestreicher N, Quesenberry CP Jr, Habel LA (2008) Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN). Breast Cancer Res Treat 112(1):165–174
Brand JS, Czene K, Eriksson L, Trinh T, Bhoo-Pathy N, Hall P, Celebioglu F (2013) Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS One 8(12):e81876
Clemons JH, Allan LM, Marvin CH, Wu Z, McCarry BE, Bryant DW, Zacharewski TR (1998) Evidence of estrogen- and TCDD-Like activities in crude and fractionated extracts of PM10 air particulate material using in vitro gene expression assays. Environ Sci Technol 32:1853–1860
Beulens JW, Monninkhof EM, Verschuren WM, van der Schouw YT, Smit J, Ocke MC, Jansen EH, van Dieren S, Grobbee DE, Peeters PH, Bueno-de-Mesquita HB (2010) Cohort profile: the EPIC-NL study. Int J Epidemiol 39(5):1170–1178
Riboli E, Kaaks R (1997) The EPIC project: rationale and study design. European prospective investigation into cancer and nutrition. Int J Epidemiol 26(Suppl 1):S6–S14
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-de-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R (2002) European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Pub Health Nutr 5(6B):1113–1124
Boker LK, van Noord PA, van der Schouw YT, Koot NV, Bueno-de-Mesquita HB, Riboli E, Grobbee DE, Peeters PH (2001) Prospect-EPIC Utrecht: study design and characteristics of the cohort population. European prospective investigation into cancer and nutrition. Eur J Epidemiol 17(11):1047–1053
Lokate M, Stellato RK, Veldhuis WB, Peeters PH, van Gils CH (2013) Age-related changes in mammographic density and breast cancer risk. Am J Epidemiol 178(1):101–109
Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH (2007) No relationship between circulating levels of sex steroids and mammographic breast density: the prospect-EPIC cohort. Breast Cancer Res 9(4):R53
Statistics Netherlands. http://www.cbs.nl/en-GB/menu/home/default.htm. Accessed 1 August 2014
Statistics Netherlands. http://www.cbs.nl/nl-NL/menu/methoden/begrippen/default.htm?ConceptID=658. Accessed 1 Aug 2014
Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dedele A, Dons E, de Nazelle A, Dimakopoulou K, Eriksen K, Falq G, Fischer P, Galassi C, Grazuleviciene R, Heinrich J, Hoffmann B, Jerrett M, Keidel D, Korek M, Lanki T, Lindley S, Madsen C, Molter A, Nador G, Nieuwenhuijsen M, Nonnemacher M, Pedeli X, Raaschou-Nielsen O, Patelarou E, Quass U, Ranzi A, Schindler C, Stempfelet M, Stephanou E, Sugiri D, Tsai MY, Yli-Tuomi T, Varro MJ, Vienneau D, Klot S, Wolf K, Brunekreef B, Hoek G (2012) Development of Land Use Regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–111205
Beelen RMJ, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai MY, Künzli N, Schikowski T, Marcon A, Eriksen K, Raaschou-Nielsen O, Stephanou E, Patelarou E, Lanki T, Yli-Tuomi T, Declercq C, Falq G, Stempfelet M, Birk M, Cyrys J, von Klot S, Nador G, Varro MJ, Dedele A, Grazuleviciene R, Molter A, Lindley S, Madsen C, Cesaroni G, Ranzi A, Badaloni C, Hoffmann B, Nonnemacher M, Kramer U, Kuhlbusch T, Cirach M, de Nazelle A, Nieuwenhuijsen M, Bellander T, Korek M, Olsson D, Stromgren M, Dons E, Jerrett M, Fischer P, Wang M, Brunekreef B, de Hoogh K (2013) Development of NO2 and NO x land use regression models for estimating air pollution exposure in 36 study areas in Europe: the ESCAPE project. Atmos Environ 72:10–23
Eeftens M, Tsai MY, Ampe C, Anwander B, Beelen R, Bellander T, Cesaroni G, Cirach M, Cyrys J, de Hoogh K, de Nazelle A, de Vocht F, Declercq C, Dedele A, Eriksen K, Galassi C, Grazuleviciene R, Grivas G, Heinrich J, Hoffmann B, Iakovides M, Ineichen A, Katsouyanni K, Korek M, Kramer U, Kuhlbusch T, Lanki T, Madsen C, Meliefste K, Molter A, Mosler G, Nieuwenhuijsen M, Oldenwening M, Pennanen A, Probst-Hensch N, Quass U, Raaschou-Nielsen O, Ranzi A, Stephanou E, Sugiri D, Udvardy O, Vaskovi E, Weinmayr G, Brunekreef B, Hoek G (2012) Spatial variation of PM2.5, PM10, PM2.5 absorbance and PM coarse concentrations between and within 20 European study areas and the relationship with NO2: results of the ESCAPE project. Atmos Environ 62:303–317
Cyrys J, Eeftens M, Heinrich J, Ampe C, Armengaud A, Beelen R, Bellander T, Beregszaszi T, Birk M, Cesaroni G, Cirach M, de Hoogh K, de Nazelle A, de Vocht F, Declercq C, Dedele A, Dimakopoulou K, Eriksen K, Galassi C, Grauleviciene R, Grivas G, Gruzieva O, Hagenbjork Gustafsson A, Hoffmann B, Iakovides M, Ineichen A, Kramer U, Lanki T, Lozano P, Madsen C, Meliefste K, Modig L, Molter A, Mosler G, Nieuwenhuijsen M, Nonnemacher M, Oldenwening M, Peters A, Pontet S, Probst-Hensch N, Quass U, Raaschou-Nielsen O, Ranzi A, Sugiri D, Stephanou E, Taimisto P, Tsai MY, Vaskovi E, Villani S, Wang M, Brunekreef B, Hoek G (2012) Variation of NO2 and NO x concentrations between and within 36 European study areas: results from the ESCAPE study. Atmos Environ 62:374–390
Lokate M, van Duijnhoven FJ, van den Berg SW, Peeters PH, van Gils CH (2013) Early life factors and adult mammographic density. Cancer Causes Control 24(10):1771–1778
American College of Radiology (ACR) (2013) American College of Radiology (ACR) Breast Imaging Reporting and Data System Atlas (BIRADS atlas), 5th edn. American College of Radiology, Reston
Kerlikowske K (2007) The mammogram that cried Wolfe. N Engl J Med 356(3):297–300
Zar JH (1999) The square root transfomation. In: Biostatistical analysis. 4th edn. Prentice-Hall, Upper Saddle River, pp 275–278
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AP (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808
Aitken Z, Walker K, Stegeman BH, Wark PA, Moss SM, McCormack VA, dos Santos Silva I (2010) Mammographic density and markers of socioeconomic status: a cross-sectional study. BMC Cancer 10:35
Beelen RMJ, Hoek G, Fischer P, van den Brandt PA, Brunekreef B (2007) Estimated longterm outdoor air pollution concentrations in a cohort study. Atmos Environ 41(7):1343–1358
Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, Forastiere F (2012) Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 11:48
Gulliver J, Morris C, Lee K, Vienneau D, Briggs D, Hansell A (2011) Land use regression modeling to estimate historic (1962–1991) concentrations of black smoke and sulfur dioxide for Great Britain. Environ Sci Technol 45(8):3526–3532
Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G (2011) Stability of measured and modelled spatial contrasts in NO(2) over time. Occup Environ Med 68(10):765–770
Velders GJM, Matthijsen J (2009) Meteorological variability in NO2 and PM10 concentrations in the Netherlands and its relation with EU limit values. Atmos Environ 43:3858–3866
Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H (1993) Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect 101(5):372–377
Zhang H, Chai Z, Sun H (2007) Human hair as a potential biomonitor for assessing persistent organic pollutants. Environ Int 33(5):685–693
Plesnicar A, Druzina B, Kovac V, Kralj B (2002) Environment and breast cancer: the role of xenooestrogens in breast cancer carcinogenesis. Radiol Oncol 36(1):63–72
Safe SH (1997) Xenoestrogens and breast cancer. N Engl J Med 337(18):1303–1304
Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7(12):1133–1144
Hall SA, Kaufman JS, Millikan RC, Ricketts TC, Herman D, Savitz DA (2005) Urbanization and breast cancer incidence in North Carolina, 1995–1999. Ann Epidemiol 15(10):796–803
Kelsey JL, Horn-Ross PL (1993) Breast cancer: magnitude of the problem and descriptive epidemiology. Epidemiol Rev 15(1):7–16
Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356(3):227–236
Acknowledgments
We thank Statistics Netherlands (CBS) for data on degree of urbanization and socio-economic status, and we thank screening organization ‘Bevolkingsonderzoek Midden-West’ for making the mammograms available.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding information
MJE received funding from the Netherlands Organisation for Health Research and Development (ZonMw) under Grant agreement number: ZONMW-200320002-UMCU. The EPIC-NL study was funded by ‘Europe against Cancer’ Programme of the European Commission (SANCO); Dutch Ministry of Health, Welfare and Sports (VWS); and ZonMw. The European Study of Cohorts for Air Pollution Effects (ESCAPE) has received funding from the European Community’s Seventh Framework Program (FP7/2007-2011) under Grant agreement number: 211250.
Ethical standards
The authors declare that the experiments comply with the current Dutch laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emaus, M.J., Bakker, M.F., Beelen, R.M.J. et al. Degree of urbanization and mammographic density in Dutch breast cancer screening participants: results from the EPIC-NL cohort. Breast Cancer Res Treat 148, 655–663 (2014). https://doi.org/10.1007/s10549-014-3205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3205-2