Abstract
The objective of this work was to detail the incidence and mortality trends of invasive and in situ breast cancer (BC) in France, especially regarding the development of screening, over the 1990–2008 period. Data issued from nine population-based cancer registries were studied. The incidence of invasive BC increased annually by 0.8 % from 1990 to 1996 and more markedly by 3.2 % from 1996 to 2003, and then sharply decreased until 2006 (−2.3 % per year), especially among women aged 50–69 years (−4.9 % per year). This trend was similar whatever the introduction date of the organized screening (OS) program in the different areas. The incidence of ductal carcinoma in situ steadily increased between 1990 and 2005, particularly among women aged 50–69 years and 70 and older. At the same time, the mortality from BC decreased annually by 1.1 % over the entire study period. This decrease was more pronounced in women aged 40–49 and 50–69 and, during the 1990–1999 period, in the areas where OS began in 1989–1991. The similarity in the incidence trends for all periods of implementation of OS in the different areas was striking. This suggests that OS alone does not explain the changes observed in incidence rate. Our study highlights the importance of closely monitoring the changes in incidence and mortality indicators, and of better understanding the factors causing variation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer-related death in women in France, as in many developed countries [1]. Many changes were observed in BC incidence trends during the past 20 years. Among the main factors that can influence BC incidence, the effects of screening and of hormone replacement therapy (HRT) after menopause are controversial, especially regarding the major decrease observed in many Western countries around 2003 [2, 3]. Other known risk factors, including endogenous and exogenous hormonal factors (e.g., age at menarche, at menopause, and at first pregnancy, lactation), alcohol consumption, obesity, genetic factors, and irradiation may also influence the incidence of BC [4].
In France, an organized screening (OS) program for BC was introduced in a few pilot areas from 1989, and then gradually extended to the whole French territory until 2004. Women aged 50–69 years (up to 74 years after 2002) were invited to undergo bilateral mammogram screening every 2 years, with 100 % reimbursement by their health insurance [5]. Alongside the OS, opportunistic screening was also performed through practitioners’ direct prescription outside the OS program [6].
Here, we detail the incidence and mortality trends of invasive and in situ BC through French population-based cancer registries data, especially regarding the development of screening.
Method
Data
This study was conducted within the framework of the French network of cancer registries (FRANCIM). Incidence analysis was based on the databases of nine registries (Bas-Rhin, Calvados, Côte d’Or, Doubs, Hérault, Isère, Loire-Atlantique, Vendée, and Tarn), selected because of their long-term routine recording of ductal carcinoma in situ (DCIS). These nine registries covered an area of 7.3 million inhabitants in 2008. All first-incident invasive female breast carcinoma and first-incident DCIS, diagnosed between 1990 and 2008, were considered for this study. Noninvasive lobular neoplasia and nonepithelial cancers, such as lymphoma and sarcoma, were excluded. French cancer registries routinely collect cases from pathological laboratories, departments of medical data processing, clinical services of public and private hospitals, health insurance funds, and medical practitioners. Data are recorded according to the European Network of Cancer Registries (ENCR) recommendations [7]. The quality and completeness of these population-based registries are certified every 4 years by an audit of the French National Institute of Health and Medical Research (Inserm) and the French Institute for Public Health Surveillance (InVS). Mortality data for BC between 1990 and 2008 were provided for each year by the Centre for Epidemiological Research into Medical Causes of Death (CépiDc).
Analysis
The incidence rates were calculated for the 19-year period (1990–2008), and based on person-years derived from the annual estimates of population by age and sex provided by the National Institute of Statistics and Economic Studies (Insee). Age-standardized rates (ASR) were estimated by the direct method using the standard world population for both incidence and mortality. Time trends in incidence and mortality were estimated using the conventional average annual percent of change (AAPC) calculated by quasi-Poisson age-period models. The setup date of screening programs varied from district to district: 1989–1991 in Bas-Rhin and Isère; 1996–1999 in Calvados, Loire-Atlantique, and Hérault; 2003–2004 in Côte d’Or, Doubs, Tarn, and Vendée. Thus, AAPCs for incidence were detailed according to four periods (1990–1996, 1996–2003, 2003–2006, and 2006–2008). Stratified analyses were conducted according to four age groups: less than 40, 40–49, 50–69, and 70 years and older. In the 50–69 years group (age group targeted by the OS program for most of the study period), stratified analyses were also conducted according to three area groups, merged by the date of initiation of the OS program (1989–1991: Bas-Rhin and Isère; 1996–1999: Calvados, Loire-Atlantique, and Hérault; 2003–2004: Côte d’Or, Doubs, Tarn, and Vendée). AAPCs for mortality were calculated for two periods (1990–1999 and 1999–2008) according to the graphical presentation of standardized mortality rate, which showed a small change in the linear trend of mortality only in 1999. Similarly to incidence, analyses were stratified according to the same age groups and area groups.
All statistical analyses were performed using R software version 3.0.1 [8].
Results
Incidence
Between 1990 and 2008, 8,232 DCIS and 78,268 invasive BC cases were diagnosed in the nine areas (Table 1). DCIS accounted for 9.5 % of all BC, for 11.5 % in women aged 40–69, but for only 5 % in women aged 70 years and older.
As shown in Fig. 1, the aged-standardized incidence rate of invasive BC annually increased moderately by 0.8 % from 1990 (ASR = 74.1/100,000) to 1996, and then sharply by 3.2 % until 2003 (ASR = 102.5/100,000). Afterward, the incidence rate of invasive BC suddenly dropped by 2.3 % per year until 2006 when it is stabilized (ASR = 94/100,000). Recent changes concerned mainly women aged 50–69 years (annual increase of 4.6 % from 1996 to 2003 and decrease of 4.9 % after 2003). From 1996 to 2003, a significant rise was also observed in women younger than 50 years but to a lesser extent (2.3 and 1.4 %, respectively, for age groups 20–39 and 40–49), and after 2003, the decrease was not significant. In women aged 70 and older, a significant annual increase of 2.0 % was observed from 1996 to 2003.
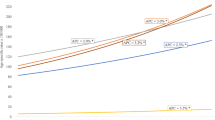
By grouping areas according to the implementation date of the OS program, the major increase observed after 1996 in women aged 50–69 years occurred in every area, including those areas that did not yet enjoy a screening program at that time (Fig. 2). Annual increases of 4.6, 4.2, and 7.4 % were seen in the areas with an OS program introduced in 1989–1991, 1996–1999, and 2003–2004, respectively. Regardless of the screening program’s date of introduction, the decrease after 2003 was similar in the three grouped areas.
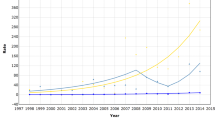
The standardized incidence rate of DCIS was twice as high in 2005 when it peaked (ASR = 14.2/100,000) as in 1990 (ASR = 7.2/100,000), before declining to 11.5/100,000 in 2008 (Fig. 3). The rise was quite substantial, being above 5 % per year between 1990 and 2003, and slowing down after 2003. Conversely to invasive BC, the annual increase over the 1996–2003 period was high in both women aged 50–69 and 70 years and older (6.1 and 8.9 %, respectively). A non-significant increase was also observed in younger women (Fig. 3). In parallel, the proportion of DCIS cases increased in each age group (Table 1).
Mortality
Between 1990 and 2008, 22,709 deaths from BC occurred in the nine study areas. During the same period, the age-standardized mortality rate annually decreased by 1.1 % from 20.1/100,000 in 1990 to 16.1/100,000 in 2008 (Fig. 4). For all women aged 40 years and over, the rate of mortality decline diminished by age group: from 2.1 % for women aged 40–49 years to 0.8 % for those aged 70 years or more. Mortality rates remained constant for women younger than 40 years, at around 1/100,000.
Among women aged 50–69 years, a significant decrease was observed between 1990 and 2008, regardless of the OS date of introduction (Fig. 5). The largest annual decrease (–2.4 %) was found in areas where OS was implemented in 1989–1991. The decrease was higher before than after 1999 (–3.7 and –2.5 %, respectively). Conversely, the mortality rate remained constant between 1990 and 1999, and then annually decreased significantly by 1.9 % until 2008 in areas that began OS in 1996–1999 as well as in 2003–2004.
Discussion
Our study provides an update of BC epidemiology using population-based data. Incidence trends of in situ carcinoma in France are provided for the first time.
Incidence
The upward trend of aged-standardized incidence rates for invasive BC observed in our study, by 0.8 % per year between 1990 and 1996 and by 3.2 % per year between 1996 and 2003, is concordant with the national estimate of a 2.4 % annual increase between 1980 and 2005 [9]. Both management practices and risk factors can influence BC incidence. Implementation of a screening program should lead to an increase in incidence in the early years, through the diagnosis of prevalent cases, then a decrease during a phase of “screening saturation” (after all prevalent cases have been discovered), followed by a stabilization and a return to the tendency induced by changes in the risk factors.
The increase in incidence was sharpest in the age group targeted by OS, supporting the idea that the introduction of OS has an impact. However, the similar patterns of incidence changes regardless of the date of OS introduction, and the rising incidence in the other age groups, suggest that the impact of the OS program itself was only partial. Part of the rise could be related to opportunistic screening, which increased in the early 1990s in France. Few data are available to estimate the widespread use of opportunistic screening. In 1994, the percentage of women aged 50–69 years, who had one mammography examination within the three previous years, ranged from 57 to 78 % (average 68 %) in five areas with an implemented OS program, and 48 % in an area without an OS program [10].
Part of the increase in incidence observed after the screening implementation could reflect overdiagnosis. A recent review of studies evaluating the proportion of cancers overdiagnosed by screening in Europe reported a value between 1 and 10 % [11]; however, this value is still debated [12], especially because of methodological reasons [11, 13, 14]. Part of the increase in incidence possibly linked to this phenomenon was too complex to consider in our study.
It is also plausible that part of the increase in the incidence of invasive BC observed from 1991 to 2003 was related to changes in exposure to risk factors. A study conducted in two areas (Isère and Tarn) highlighted an increase in the incidence of both cancers of advanced stages (T3–4 or N1 or M1) and of localized stages between 1990 and 2003 in women aged 50–74 years [15]. The growing use of HRT in the 1990s in France may have played a role in the inflation of BC incidence together with the development of screening. It is likely that other risk factors (such as reduced parity or increasing age at first pregnancy for women born between 1935 and 1950) have also impacted incidence. Indeed, a previous work showed that BC incidence began to rise prior to 1990 and to the implementation of a screening program [9].
After 2003, the incidence of invasive BC decreased by 2.3 % per year until 2006. As in many countries in the early 2000s, this occurred particularly among women aged 50–65 years [16, 17]. There has been much debate on the reason for the decline [2, 3, 18]. It could be related to the coexistence of several factors: a decrease in the prescription of HRT combined with a possible saturation of screening. In the USA, BC incidence started to decrease before 2000, slowly until 2002 (possibly related to screening saturation) and then more sharply after 2002, with the additional effect of decreasing HRT use [19]. By contrast, in France, the screening program was introduced gradually and generalized only in 2004; however, opportunistic screening increased from the early 1990s. In our study, the similarity in incidence trends of invasive cancers suggests that the saturation phase, combining opportunistic and OS, occurred at exactly the same time in all the areas where OS was implemented at different periods. This would be quite surprising. It is more likely that the rapid decline in HRT use of 62 % from 2001 to 2006, evidenced by the National Health Fund, sharpest in women aged 50–64 years between 2002 and 2004, partly explained the drop in BC incidence in 2003, in addition to a partial screening saturation effect in some areas [20]. The effect of HRT should be temporary, because it only exerts a promoter effect accelerating the growth of already existing tumor cells [21].
Stabilization or a decrease of other known risk factors, such as the significant drop in alcohol consumption between 1955 and 2008, should also be mentioned [22]. However, for younger cohorts of women, other factors seem to develop rather unfavorably, such as increasing age at first pregnancy, obesity, or night work. These factors change gradually over time, and if they continue to develop adversely in the next few years, then the incidence could start increasing again in women aged 40–74, as already evidenced in women younger than 40 years [23].
The incidence of DCIS annually rose by 5.3 % between 1990 and 1996 and then by 5.7 % between 1996 and 2003. This increase was similar to the increase in the proportion of DCIS among BC cases: from 7.6 % in 1990–1992 to 13.5 % in 2005–2008 among women aged 50–69 years. The incidence trend of DCIS did not completely overlap that of invasive BC. It was consistent with the development of screening practices as described in other countries [24–26]. DCIS incidence in France was close to that observed in the UK but was lower than that observed in the US, where the ratio DCIS/BC exceeded 20 % in 2005 [25, 27]. According to Kumar et al., differences in DCIS/BC proportion between the US and the UK were partly attributed to the differences in diagnosis practices (interpretation of mammograms and recall rate, use of open biopsy, and positive predictive value of open biopsy) [27]. Part of the increase may reflect overdiagnosis [14]. However, the increase of DCIS both in incidence rates and in the proportion of diagnosed cancers occurring in women younger than 50 and older than 69 years was also in favor of improvements in medical practices and in radiological diagnostic technologies. It was evidenced that the detection of DCIS is greatest at baseline screening than at subsequent screenings [25]. Thus, the decline after 2005 could correspond to a saturation of individual and organized combined screening. The incidence trends of DCIS may better reflect screening expansion compared with those of invasive BC.
Mortality
We found a significant average annual decrease of 1.1 % in BC mortality in the study area from 1990 to 2008. The annual decrease of 1.5 % during 1999–2008 was close to the French national estimate of 1.5 % over the 1999–2004 period [28]. This is consistent with the reduction of mortality observed in many Western countries during recent decades [28, 29].
The impact of the implementation of screening programs is still debated. Numerous studies have demonstrated that the early detection of tumors can reduce mortality. The latest meta-analyses found a reduction in mortality from 15 to 20 % in favor of screening [30, 31], and the recent large-scale population studies a reduction of mortality for screening from 6.4 to 25 % [32–34].
Some patterns of the decline in mortality from BC observed in our study were in favor of an impact of OS: the reduction accelerated after 1999, when five of the nine areas were covered by OS; although not the greatest one, a reduction was found in women aged 50–69 years, the targeted age for OS, and the largest decrease was observed in areas that established OS in 1989–1991. This reduction was rather modest in comparison with other European countries where a decline of 1 to 9 % was observed before and at least 10 years after the introduction of screening [35].
By contrast, several facts did not support the impact of OS alone on the mortality decline in our study. We found a decrease twice as high among women aged 40–49 years as compared with women aged 50–69 years. The same changes in mortality rate in the areas that started OS in 2003–2004 occurred as early (in 1999), as in those that started before. Furthermore, we observed a significant decrease in rates shortly after implementation of OS, although the effects on mortality do not usually appear immediately, but several years after OS implementation [36].
Part of the decrease in mortality could be attributed to advances in therapy, which dramatically improved in last few decades. The contribution of screening on the mortality reduction observed between 1975 and 2000 in the United States was estimated between 28 and 65 % (median contribution 46 %) [37]. In Norway, Kalager et al. reported that screening is only responsible for 33 % of the estimated reduction in mortality [32]. Some authors stressed the potentiating effects of both early detection and appropriate care on the reduction of BC mortality [32, 38, 39]. In Catalonia, among women aged 30–69 years, the reduction in BC mortality between 1975 and 2008 was estimated to be due to screening for 20.4 %, to adjuvant therapy for 15.8 %, and to a combination of both factors for 34.1 % [38]. In the United States, the percentage of reduction in mortality from BC between 1975 and 2000 was 7.5–22.7 % for detection, 12–20.8 % for adjuvant treatment [39], and 24.9 % for the combination of both [40].
As in many studies on recent trends [41], we have not been able to demonstrate a direct relationship between the gradual establishment of an OS program and the reduction in BC mortality in several French areas. The only French study aimed at evaluating the effect of BC screening on related mortality estimated a 19–23 % reduction in mortality for cancer diagnosed between 1990 and 1996 [42]. Our results were clearly in favor of the important role played by improvements in therapeutic management. The impact of OS on mortality may not be entirely visible yet because the last areas only began the program in 2004, and opportunistic screening makes it difficult to interpret mortality trends in France.
The strength of the present study lies in the standardized procedures of data collection and coding used by all French cancer registries over long periods. Nevertheless, the study may have some limitations. The areas studied may not be representative of the whole of France. However, the diversity of situations in terms of population covered and of OS practices, and the similarity of trends in all areas, argue in favor of the relevance of our results. Moreover, estimated trends in incidence and mortality at a national level were consistent with ours. Another limitation is that descriptive epidemiology alone cannot measure the impact of the changes in known risk factors or medical practices (including OS) on incidence and mortality trends. Although the results should be interpreted cautiously, this study allows many assumptions to be made.
One impressive result was the similarity of incidence trends in the different areas for all the periods of OS implementation. As pointed out by other authors, the observed changes in incidence rates certainly reflect the combination of several phenomena [43, 44]. Long-term incidence trends related to a progressive change in exposure to certain risk factors (such as reproductive patterns) overlap with temporary short-term fluctuations related to the fast changes in other risk factors such as screening or HRT use. Our study highlights the importance of closely monitoring the changes in incidence and mortality indicators, and of better understanding the factors causing variation.
References
Forouzanfar MH, Foreman KJ, Delossantos AM et al (2011) Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 378:1461–1484. doi:10.1016/S0140-6736(11)61351-2
Glass AG, Lacey JV Jr, Carreon JD, Hoover RN (2007) Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 99:1152–1161. doi:10.1093/jnci/djm059
Weedon-Fekjær H, Bakken K, Vatten LJ, Tretli S (2012) Understanding recent trends in incidence of invasive breast cancer in Norway: age-period-cohort analysis based on registry data on mammography screening and hormone treatment use. BMJ 344:e299
Key TJ, Verkasalo PK, Banks E (2001) Epidemiology of breast cancer. Lancet Oncol 2:133–140. doi:10.1016/S1470-2045(00)00254-0
Bulletin Officiel No. 2001-43. http://www.sante.gouv.fr/fichiers/bo/2001/01-43/a0432846.htm. Accessed Nov 25 2013
Duport N (2012) Characteristics of women using organized or opportunistic breast cancer screening in France. Analysis of the 2006 French Health, Health Care and Insurance Survey. Rev Epidémiol Sante Publique 60:421–430. doi:10.1016/j.respe.2012.05.006
European Network of Cancer Registries, Tyczyński JE, Démaret E, et al (2003) Standards and guidelines for cancer registration in Europe: the ENCR recommendations: volume I. International Agency for Research on Cancer, Lyon
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Belot A, Grosclaude P, Bossard N et al (2008) Cancer incidence and mortality in France over the period 1980–2005. Rev Epidémiologie Santé Publique 56:159–175. doi:10.1016/j.respe.2008.03.117
Lacour A, Mamelle N, Arnold F et al (1997) Mass screening programs for breast cancer in France. Comparative evaluation. J Gynécologie Obstétrique Biol Reprod 26:470–483
Puliti D, Duffy SW, Miccinesi G et al (2012) Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 19(Suppl 1):42–56. doi:10.1258/jms.2012.012082
Marmot MG (2013) Sorting through the arguments on breast screening. JAMA 309:2553–2554. doi:10.1001/jama.2013.6822
Wu D, Pérez A (2011) A limited review of over diagnosis methods and long term effects in breast cancer screening. Oncol Rev 5:143–147. doi:10.1007/s12156-011-0077-0
De Gelder R, Heijnsdijk EAM, van Ravesteyn NT et al (2011) Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev 33:111–121. doi:10.1093/epirev/mxr009
Daubisse-Marliac L, Delafosse P, Boitard JB et al (2011) Breast cancer incidence and time trend in France from 1990 to 2007: a population-based study from two French cancer registries. Ann Oncol 22:329–334. doi:10.1093/annonc/mdq396
Ravdin PM, Cronin KA, Howlader N et al (2007) The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356:1670–1674. doi:10.1056/NEJMsr070105
Zbuk K, Anand SS (2012) Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health 66:1–7. doi:10.1136/jech.2008.083774
Gompel A, Plu-Bureau G (2010) Is the decrease in breast cancer incidence related to a decrease in postmenopausal hormone therapy? Ann N Y Acad Sci 1205:268–276. doi:10.1111/j.1749-6632.2010.05664.x
Jemal A, Ward E, Thun MJ (2007) Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res 9:R28. doi:10.1186/bcr1672
Séradour B, Allemand H, Weill A, Ricordeau P (2009) Changes by age in breast cancer incidence, mammography screening and hormone therapy use in France from 2000 to 2006. Bull Cancer 96:E1–E6. doi:10.1684/bdc.2009.0869
Dietel M, Lewis MA, Shapiro S (2005) Hormone replacement therapy: pathobiological aspects of hormone-sensitive cancers in women relevant to epidemiological studies on HRT: a mini-review. Hum Reprod (Oxford, England) 20:2052–2060. doi:10.1093/humrep/dei043
Hill C, Laplanche A (2010) La consommation d’alcool est trop élevée en France. Presse Médicale 39:e158–e164. doi:10.1016/j.lpm.2009.12.010
Leclère B, Molinié F, Trétarre B et al (2013) Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol 37:544–549. doi:10.1016/j.canep.2013.05.001
Sørum R, Hofvind S, Skaane P, Haldorsen T (2010) Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast (Edinburgh, Scotland) 19:499–505. doi:10.1016/j.breast.2010.05.014
Virnig BA, Wang S-Y, Shamilyan T et al (2010) Ductal carcinoma in situ: risk factors and impact of screening. JNCI Monogr 2010:113–116. doi:10.1093/jncimonographs/lgq024
Toriola AT, Colditz GA (2013) Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat 138:665–673. doi:10.1007/s10549-013-2500-7
Kumar AS, Bhatia V, Henderson IC (2005) Overdiagnosis and overtreatment of breast cancer: rates of ductal carcinoma in situ: a US perspective. Breast Cancer Res 7:271–275. doi:10.1186/bcr1346
Autier P, Boniol M, La Vecchia C et al (2010) Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ 341:c3620. doi:10.1136/bmj.c3620
Broeders M, Moss S, Nyström L et al (2012) The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen 19(Suppl 1):14–25. doi:10.1258/jms.2012.012078
Independent UK Panel (2012) The benefits and harms of breast cancer screening: an independent review. Lancet 380:1778–1786. doi:10.1016/S0140-6736(12)61611-0
Gøtzsche PC, Nielsen M (2011) Screening for breast cancer with mammography. Cochrane Database Syst Rev (1):CD001877. doi:10.1002/14651858.CD001877.pub4
Kalager M, Zelen M, Langmark F, Adami H-O (2010) Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med 363:1203–1210. doi:10.1056/NEJMoa1000727
Blanks RG, Moss SM, McGahan CE et al (2000) Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ 321:665–669
Olsen AH, Njor SH, Lynge E (2007) Estimating the benefits of mammography screening: the impact of study design. Epidemiology (Cambridge, Mass.) 18:487–492. doi:10.1097/EDE.0b013e318060cbbd
Moss SM, Nyström L, Jonsson H et al (2012) The impact of mammographic screening on breast cancer mortality in Europe: a review of trend studies. J Med Screen 19(Suppl 1):26–32. doi:10.1258/jms.2012.012079
Hanley JA (2011) Measuring mortality reductions in cancer screening trials. Epidemiol Rev 33:36–45. doi:10.1093/epirev/mxq021
Berry DA, Cronin KA, Plevritis SK et al (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792. doi:10.1056/NEJMoa050518
Vilaprinyo E, Puig T, Rue M (2012) Contribution of early detection and adjuvant treatments to breast cancer mortality reduction in Catalonia, Spain. PLoS One 7:e30157. doi:10.1371/journal.pone.0030157
Lee S, Zelen M (2006) A stochastic model for predicting the mortality of breast cancer. J Natl Cancer Inst Monogr (36):79–86. doi:10.1093/jncimonographs/lgj011
Mandelblatt J, Schechter CB, Lawrence W, et al (2006) The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr (36):47–55. doi:10.1093/jncimonographs/lgj008
Youlden DR, Cramb SM, Dunn NAM et al (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36:237–248. doi:10.1016/j.canep.2012.02.007
Uhry Z, Hédelin G, Colonna M et al (2011) Modelling the effect of breast cancer screening on related mortality using French data. Cancer Epidemiol 35:235–242. doi:10.1016/j.canep.2010.10.009
Sprague BL, Trentham-Dietz A, Remington PL (2011) The contribution of postmenopausal hormone use cessation to the declining incidence of breast cancer. Cancer Causes Control CCC 22:125–134. doi:10.1007/s10552-010-9682-7
Ringa V, Fournier A (2008) Did the decrease in use of menopausal hormone therapy induce a decrease in the incidence of breast cancer in France (and elsewhere)? Rev Epidémiologie Santé Publique 56:297–301. doi:10.1016/j.respe.2008.07.085
Acknowledgments
Grant sponsor: National Cancer Institute (INCa), French Institute for Public Health Surveillance (InVS). We are grateful for the generous assistance given by the pathologists, oncologists, Departments of Medical Data Processing of public and private hospitals, and medical practitioners, regarding access to medical records.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molinié, F., Vanier, A., Woronoff, A.S. et al. Trends in breast cancer incidence and mortality in France 1990–2008. Breast Cancer Res Treat 147, 167–175 (2014). https://doi.org/10.1007/s10549-014-3073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3073-9