Abstract
The purpose is to examine the effects of melatonin supplementation on sleep, mood, and hot flashes in postmenopausal breast cancer survivors. In a randomized, double-blind, placebo-controlled study, 95 postmenopausal women with a prior history of stage 0–III breast cancer, who had completed active cancer treatment (including hormonal therapy) were randomly assigned 1:1 to either 3 mg oral melatonin (n = 48) or placebo daily (n = 47) for 4 months. Sleep, mood, and hot flashes were assessed at baseline and 4 months via self-administered questionnaire using the Pittsburgh Sleep Quality Index (PSQI), Center for Epidemiologic Studies—Depression (CES-D), and the North Central Cancer Treatment Group (NCCTG) hot flash diary, respectively. Eighty-six women (91 %) completed the study and provided pre- and post-questionnaires. At baseline, 52 % of participants reported poor sleep in the month prior to enrollment. Compared to subjects on placebo, subjects randomized to melatonin experienced significantly greater improvements in subjective sleep quality as measured by the PSQI, including domains on sleep quality, daytime dysfunction and total score. For example, the mean change in PSQI score was −0.1 in the placebo group compared to −1.9 in the melatonin group (p < 0.001). There were no significant differences in measures of depression or hot flashes. Sleep disturbances are common among breast cancer survivors, even after completion of active cancer treatment. This is the first randomized placebo-controlled study among breast cancer survivors to demonstrate that melatonin was associated with an improvement in subjective sleep quality, without any significant adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep disturbances are common among breast cancer survivors [1]. Melatonin has been widely evaluated as treatment for jet lag and insomnia [2, 3]. More limited evidence also suggests a potential role of melatonin supplements and its analogs in the treatment of depression [4]. Additionally, it has been observed that melatonin levels decrease with age, particularly around menopause, so may affect hot flashes [5, 6]. Melatonin is available over the counter and has been extensively evaluated in humans where it does not seem to have any significant adverse effects across a wide range of doses and periods of use [3, 7, 8].
In order to evaluate the impact of melatonin on a variety of breast cancer biomarkers as well as quality of life endpoints, we conducted a double-blind, placebo-controlled, randomized trial among breast cancer survivors at the Dana-Farber Cancer Institute (DFCI). We had previously published our results on breast cancer biomarkers which were the primary endpoints for the study [9]. We now present the results for the quality-of-life endpoints which were secondary endpoints.
Methods
Patients
Eligible subjects included postmenopausal women with a history of a primary breast cancer (including Stages I–III), ductal carcinoma in situ, or lobular carcinoma in situ and had completed all active cancer treatment including surgery, radiation, chemotherapy, and hormonal therapy at least 60 days prior to enrollment. Exclusion criteria included metastatic breast cancer, history of prior malignancies other then breast cancer or non-melanoma skin cancer, regular overnight shift work (defined as >1 overnight shift per month), active seizure disorder requiring daily anti-epileptic medication, or concomitant use of beta-blockers, warfarin, menopausal hormone therapy, black cohosh/flaxseed/soy supplements, or regular nightly use of sleep aids. No melatonin supplement use was allowed within 30 days prior to enrollment or while on study. Written informed consent was obtained from all participants before study entry. The protocol was approved by the Internal Review Board of the Dana Farber Harvard Cancer Center.
Study treatment
Study subjects were randomly assigned (1:1) to receive either 4 months of 3 mg melatonin or placebo taken nightly at 9 p.m. due to melatonin’s possible sedating effect. The study was double-blinded and only the pharmacists had access to the assigned treatment. Subjects were dispensed the study drug on the same day that they were randomized and provided their baseline blood sample and were instructed to start the study drug that evening. For verification of compliance, each subject completed a medication diary and pill counts were also done. Missed doses were not made up and there were no provisions for dose reductions. Melatonin supplements were purchased from Rugby Laboratories, a subsidiary of Watson Laboratories (Duluth, GA, USA). For each lot of melatonin, Rugby Laboratories issued a certificate of analysis guaranteeing the composition and purity. The DFCI Investigational Drug pharmacy prepared identical gelatin blue capsules with either melatonin and methycellulose filler or methylcellulose filler alone as placebo.
Statistical methods
The endpoints for this analysis were changes in sleep quality, depression, and hot flashes. Sleep quality, mood, and hot flash severity were assessed at baseline and after completion of the study intervention using the Pittsburgh Sleep Quality Index (PSQI), Center for Epidemiologic Studies-Depression (CES-D) scale, and North Central Cancer Treatment Group (NCCTG) hot flash diary, respectively. The PSQI is a well-validated, self-administered, 19-item questionnaire that assesses general sleep quality over the past month [10]. It differentiates “poor” from “good” sleep by measuring seven areas: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction during the last month. Scoring is based on a 0–3 scale with a score of three reflecting the negative extreme on the Likert Scale. The scores for the seven areas are summed to determine an overall, global score. A higher score indicates worse sleep and a global score of five or greater indicates a “poor” sleeper with a high degree of sensitivity and specificity. The CES-D is a widely used 20-item questionnaire regarding depressive symptoms over the past week that has been tested in healthy subjects, as well as patients with a history of breast cancer [11, 12]. The North Central Cancer Treatment Group (NCCTG) hot flash diary measures both frequency and intensity of hot flashes over the past 7 days and has been widely used in clinical trials in breast cancer patients [13]. Subjects keep track of the number and severity of hot flashes for a week and are also asked to grade the severity of the hot flashes on a scale of 1–4 (1 = mild, 2 = moderate, 3 = severe, and 4 = very severe). Data are summarized by (a) counting the number and severity of hot flashes per day, and (b) calculating a hot flash score using both the frequency and the severity rating. For example, a subject who records 10 hot flashes a day and classifies the severity of each as 3 will have a score of 30. All analyses were done on an intention-to-treat basis. Comparisons between treatment groups were conducted using the Wilcoxon rank-sum test for continuous measures and Fisher’s exact test for categorical measures. Two-sided p values less than 0.05 were considered statistically significant. For assessments with multiple subscales, a Bonferroni correction for multiple comparisons was conducted for the subscales to maintain an assessment-wide type-I error of 0.05.
The trial was powered for the primary endpoint which was a change in estradiol and insulin-like growth factor (IGF)-1 levels. For the secondary endpoints of changes in PSQI and CES-D overall scores, we had 80 % power to detect differences in change scores over times that are 0.46 times the common standard deviation. Using the observed study data, this would translate into detecting a 1.1 point difference in PSQI score and 2.1 point difference in CED-D score. Since the baseline hot flash score was only calculated for women who reported having hot flashes in the week prior to enrollment (n = 44), we had 80 % power to detect a difference of 2.4 in average change scores.
Results
Patient characteristics and compliance
A total of 95 subjects were enrolled on the study between March 2007 and March 2009 from the Dana-Farber Cancer Institute (Boston, MA, USA). Baseline demographics overall and by treatment arms are presented in Table 1. In general, the two treatment arms were well balanced. Mean age at enrollment was 59 (range 38–80). The median time from breast cancer diagnosis to enrollment in the trial was 7.6 years (range 0.9–27.9 years). Of 95 subjects enrolled, 86 (90.5 %) completed the 4-month intervention and nine subjects (9.5 %) did not complete the intervention. The reasons for non-completion included: one subject (randomized to placebo) withdrew consent for continued participation due to insomnia because she was required to stop her regular sleep medication and suffered insomnia as a result; four subjects (all in the melatonin arm) withdrew due to toxicity (all grades 1 or 2), including headaches, insomnia, and nightmares; three subjects (two on placebo and one on melatonin) were lost to follow-up, and another subject (randomized to placebo) left the study after 10 weeks to begin radiotherapy for local breast cancer recurrence. The observed rate of attrition is 9.5 % (8.5 % placebo, 10.4 % melatonin). There was no difference in the rates of completion between the placebo and melatonin groups (Fisher’s exact p value = 0.75). We had previously reported on compliance, and it was high overall (89.5 %) and equivalent in both arms [9].
Table 2 summarizes the baseline measures overall and by treatment arm. There were no significant differences in global PSQI, CES-D, or hot flash scores between subjects assigned to placebo compared to melatonin. Based upon the cutoff of a global score of five or greater, 47 of 90 subjects (52.2 %) were poor sleepers during the month preceding the baseline assessment (42.6 % on placebo and 56.3 % on melatonin, p for difference = 0.49) [10]. For the CES-D, scores of 16 or greater indicate depression within the preceding week [12]. According to this cut point, 11 subjects (12.6 %) were depressed during the week before the baseline assessment (16.3 % placebo, 9.1 % melatonin, p for difference = 0.36). Forty-four subjects (46.3 %) reported hot flashes during the week preceding the baseline assessment, with no difference between treatment groups (p = 0.31). The number, severity, and hot flash scores for these women are summarized in Table 2.
Changes in sleep
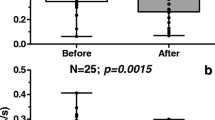
Table 3 presents the data on changes in sleep quality, mood, and hot flashes between baseline and end of treatment at 4 months. Overall, there was a general improvement in PSQI scores over all domains and subjects randomized to melatonin reported larger improvements in sleep than subjects who took placebo. After adjusting for multiple comparisons, subjects taking melatonin had significantly improved sleep quality, daytime dysfunction, and PSQI total scores when compared with subjects taking placebo. It should be noted that a higher PSQI score reflects worse sleep quality, so a positive change score (4 month—baseline) would indicate worsening sleep quality over time. Although there are no defined criteria for a “clinically significant” difference in PSQI score, our effect size was similar to that of other insomnia treatment studies [14–16] and almost equivalent to one standard deviation for the total PSQI score within our study population. As stated previously, 43 % of patients on placebo and 56 % on melatonin were classified as poor sleepers at baseline based upon the global PSQI score cutoff of five or more points. Although this difference was not significant (p = 0.49), we constructed a multivariate linear model of change in overall PSQI score using treatment, baseline sleep quality, and baseline depression as predictors. When adjusted for baseline sleep quality and depression, the change in overall PSQI for subjects assigned to placebo was 1.67 points higher (worse) than subjects taking melatonin (95 % CI 0.67–2.66, p = 0.001).
We also examined changes among subjects who were classified at baseline as “poor sleepers” based upon a global PSQI score >5 (Table 4). Even though small numbers precluded formal statistical testing, we observed a qualitatively greater improvement in overall sleep quality among poor sleepers randomized to melatonin. Specifically, in the placebo arm, only three people (7.7 %) who reported poor sleep at baseline no longer had poor sleep at 4 months and two people without a report of poor sleep at baseline became poor sleepers during the course of the study. In contrast, among people randomized to the melatonin arm, eight subjects (19.5 %) who were poor sleepers at baseline were no longer poor sleepers at 4 months, and no subjects became poor sleepers during the trial.
Changes in depression and hot flashes
For the CES-D (Table 3), there was no significant difference between average change scores for placebo and melatonin subjects. In terms of hot flashes, 44 subjects reported hot flashes at baseline. At the end of the intervention, 12 patients who completed the study no longer reported hot flashes (6 on placebo, 6 on melatonin). For calculation of the hot flash summaries, the 12 subjects who changed hot flash status by the end of the intervention were included with scores of zero. Generally, the number and severity of hot flashes decreased over time for both treatment arms. There were no statistically significant differences in change scores noted between the two treatments for any of the components of the assessed hot flash scores.
Toxicity
We have previously reported detailed toxicity data [9]. Briefly, for the melatonin arm, there were no grade 3/4 toxicities. The most common grade 1/2 toxicities for melatonin were headache, fatigue, and bad dreams.
Discussion
In this double-blind randomized controlled trial, we have demonstrated the efficacy of melatonin in improving sleep among breast cancer survivors. Melatonin was well tolerated with a high level of compliance. Importantly, for breast cancer survivors, we have previously demonstrated that melatonin does not have any adverse effects on circulating estradiol or IGF-1 levels [9]. Although there are no defined criteria for a “clinically significant” change in PSQI score, the magnitude of the effect we observed was similar to other studies of pharmacologic and lifestyle treatments of insomnia [14–16] and almost equivalent to one standard deviation for the total PSQI score within our study population. When looking specifically at poor sleepers, there appeared to be more improvement qualitatively in the melatonin arm.
Melatonin has been extensively studied in cancer patients, both alone and given with chemotherapy without any significant adverse events at a wide range of doses and durations [7, 8]. Multiple studies have demonstrated its efficacy in helping with sleep, both for delayed sleep phase disorder and jet lag, as well as primary insomnia [2, 3, 17]. Unlike our study population, most previous studies were specifically among subjects reporting sleep disturbances. Our study is the first randomized placebo-controlled study to demonstrate its efficacy and safety for sleep issues among breast cancer survivors. We had previously demonstrated that there were no significant changes in circulating estradiol, insulin-like growth factors-1, or IGF-binding protein 3 with this intervention [9]. It should be noted that the melatonin for this study was purchased from a single supplier who guaranteed the purity and composition. However, given the lack of regulation and quality control of dietary supplements in the United States, the quality of products purchased in other settings is not known.
Sleep disturbances among breast cancer survivors are common and can significantly impact quality of life [18]. The exact prevalence varies considerably from study to study depending upon the measure used to assess sleep quality, which range from questionnaire assessments to polysomnograph/actigraph measures. Importantly, sleep disturbances are common both during and long after completion of active cancer treatment [1, 18, 19]. For example, among 246 breast cancer survivors who were at least 5 years after diagnosis, 65 % were considered “poor sleepers” based upon the total PQSI score, compared to 55 % of age-matched controls without cancer (p < 0.05) [19]. Similarly in our study, even though all participants had already completed their active cancer treatment, including hormonal therapy, over 50 % were classified as “poor sleepers” according to the PSQI.
Multiple interventions have been evaluated for treatment of sleep disturbances among breast cancer survivors. Although cognitive behavioral therapy (CBT) has been shown to improve sleep in randomized controlled trials, CBT is quite resource intensive for both the patient and provider and is not widely available [20–22]. Physical activity has also been shown to improve sleep quality among cancer survivors [14, 15], but adherence to exercise regimens can be low over time. Long term use of hypnotic agents is discouraged due to habituation. Our study suggests that melatonin may provide an effective intervention for breast cancer survivors.
To our knowledge, only two other randomized trials have evaluated melatonin and menopausal symptoms. The larger study (232 women included in the final analysis) was a randomized 2 × 2 factorial design trial of (1) soy isoflavones + 3 mg melatonin, (2) soy isoflavones alone, (3) melatonin alone, or (4) placebo. For menopausal symptoms, there was no difference by treatment group [23]. The more recent study was a randomized double-blind, placebo-controlled study of 3 mg nightly melatonin versus placebo for 6 months with the primary endpoints being bone density and bone turnover markers [24]. They found an improvement in physical domain scores on the Menopause Specific Quality of Life (MENQOL) questionnaire but no differences in the vasomotor, psychosocial, or sexual domains of the MENQOL or global PSQI score, but only 18 subjects were enrolled. We found no difference in hot flash score comparing melatonin and placebo, but our trial was not powered to detect a difference in hot flashes and only 44 women reported hot flashes at baseline.
Many of the symptoms of clinical depression are similar to those of circadian disturbance (e.g., insomnia, early-morning awakening, daytime fatigue, etc.). In addition to its beneficial effects on the sleep-wake cycle, melatonin analogs may also help to reset other neurohormonal/endocrine (e.g., cortisol) circadian disruptions that accompany depression. Melatonin itself has not proven very effective in trials of treatment for clinical depression, but melatonin analogs hold considerable promise [4, 25]. We did not see an effect of melatonin on depression scores, but our subjects were not clinically depressed and our trial was not powered to detect an effect on depression.
Although the optimal dose of melatonin is not known, 3 mg per day was chosen because this results in supraphysiologic levels of melatonin at night, but should still return to normal with little hangover effect in the morning as demonstrated by jet lag and insomnia trials [2, 3], which commonly used doses ranging from 0.1 mg to 5 mg (with the higher doses usually more efficacious for sleep). Doses from 1 to 5 mg are widely available over the counter as a sleep aid and have been used safely in multiple studies. It is possible that higher doses than 3 mg melatonin may be even more effective, but doses of 0.1–5 mg are already considered supraphysiologic and studies do not tend to support an additional dose effect at higher levels. Moreover, the long-term safety of doses above 5 mg has not been evaluated in as many studies as doses between 0.1 and 5 mg doses [3, 7, 8].
For this trial, we excluded subjects on chemotherapy or hormonal therapy, so the effects of melatonin during active cancer treatment are unknown. Previous randomized trials with melatonin in combination with chemotherapy and cross-over studies with tamoxifen did not show any deleterious effects. In fact, a few studies reported improved efficacy and/or decreased toxicity with the addition of melatonin, with the caveat that these studies all came from the same group of investigators [26–29]. Both normal and neoplastic breast tissue express melatonin receptors (MT1), but expression is higher in breast cancer [30]. Melatonin can be anti-proliferative in estrogen receptor (ER) positive breast cancer cell line [31, 32] and may decrease ER expression and binding [33, 34]. Higher MT1 tumor expression has been associated with better prognosis in one study [35]. Overall, these data suggest that melatonin is unlikely to worsen clinical outcomes in patients on treatment, but this would need to be confirmed.
Limitations of our study include that we did not specifically recruit subjects with sleep disorders, so it is unclear if our results would be generalizable to subjects with more severe chronic insomnia. However, reports of sleep disturbances are common among breast cancer survivors, as was seen in our trial. Although the PSQI is a well-validated study, a more comprehensive sleep study would provide more detailed monitoring of sleep and potential areas for improvement. Our study lasted only four months, so it is unknown if the effects may become attenuated over time.
In summary, our study is the first randomized placebo-controlled trial to suggest the efficacy and safety of melatonin among breast cancer survivors. It should be noted that the primary objective of this study was a change in biomarkers and that sleep was a secondary objective. Future studies should confirm our findings and evaluate the efficacy of melatonin in helping with sleep among subjects undergoing active cancer treatment and whether the improvements in sleep quality associated with melatonin will translate into improvement in fatigue, since fatigue is a primary complaint of many breast cancer survivors.
Abbreviations
- CBT:
-
Cognitive behavioral therapy
- CES-D:
-
Center for Epidemiologic Studies-Depression
- DFCI:
-
Dana-Farber Cancer Institute
- ER:
-
Estrogen receptor
- IGF:
-
Insulin-like growth factor
- MENQOL:
-
Menopause Specific Quality of Life
- MT1:
-
Melatonin receptor 1
- NCCTG:
-
North Central Cancer Treatment Group
- PSQI:
-
Pittsburgh Sleep Quality Index
References
Fiorentino L, Ancoli-Israel S (2006) Insomnia and its treatment in women with breast cancer. Sleep Med Rev 10(6):419–429
Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G (2006) Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ 332(7538):385–393
Herxheimer A, Petrie KJ (2002) Melatonin for the prevention and treatment of jet lag. Cochrane Database of Systematic Reviews
Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (2012) Melatonin and its analogs in insomnia and depression. J Pineal Res 52(4):365–375
Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E (2001) Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp Gerontol 36(2):297–310
Iguichi H, Kato KI, Ibayashi H (1982) Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab 55(1):27–29
Jung B, Ahmad N (2006) Melatonin in cancer management: progress and promise. Cancer Res 66(20):9789–9793
Mills E, Wu P, Seely D, Guyatt G (2005) Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res 39(4):360–366
Schernhammer ES, Giobbie-Hurder A, Gantman K, Savoie J, Scheib R, Parker LM, Chen WY (2012) A randomized controlled trial of oral melatonin supplementation and breast cancer biomarkers. Cancer Causes Control 23(4):609–616
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res 28(2):193–213
Hann D, Winter K, Jacobsen P (1999) Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res 46(5):437–443
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure 1:385–401
Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H (2001) Methodologic lessons learned from hot flash studies. J Clin Oncol 19(23):4280–4290
Courneya KS, Sellar CM, Trinh L, Forbes CC, Stevinson C, McNeely ML, Peddle-McIntyre CJ, Friedenreich CM, Reiman T (2012) A randomized trial of aerobic exercise and sleep quality in lymphoma patients receiving chemotherapy or no treatments. Cancer Epidemiol Biomarkers Prev 21(6):887–894
Payne JK, Held J, Thorpe J, Shaw H (2008) Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum 35(4):635–642
Yurcheshen ME, Guttuso T Jr, McDermott M, Holloway RG, Perlis M (2009) Effects of gabapentin on sleep in menopausal women with hot flashes as measured by a Pittsburgh Sleep Quality Index factor scoring model. J Womens Health (Larchmt) 18(9):1355–1360
van Geijlswijk IM, Korzilius HP, Smits MG (2010) The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep 33(12):1605–1614
Alfano CM, Lichstein KL, Vander Wal GS, Smith AW, Reeve BB, Mc Tiernan A, Bernstein L, Baumgartner KB, Ballard-Barbash R (2011) Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat 130(1):243–254
Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL (2010) Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage 39(3):535–547
Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, Agrawal S (2009) One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol 27(35):6033–6040
Epstein DR, Dirksen SR (2007) Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum 34(5):E51–E59
Savard J, Simard S, Ivers H, Morin CM (2005) Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: immunologic effects. J Clin Oncol 23(25):6097–6106
Secreto G, Chiechi LM, Amadori A, Miceli R, Venturelli E, Valerio T, Marubini E (2004) Soy isoflavones and melatonin for the relief of climacteric symptoms: a multicenter, double-blind, randomized study. Maturitas 47(1):11–20
Kotlarczyk MP, Lassila HC, O’Neil CK, D’Amico F, Enderby LT, Witt-Enderby PA, Balk JL (2012) Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res 52(4):414–426
Hickie IB, Rogers NL (2011) Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet 378(9791):621–631
Lissoni P, Barni S, Mandala M, Ardizzoia A, Paolorossi F, Vaghi M, Longarini R, Malugani F, Tancini G (1999) Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer 35(12):1688–1692
Lissoni P, Barni S, Meregalli S, Fossati V, Cazzaniga M, Esposti D, Tancini G (1995) Modulation of cancer endocrine therapy by melatonin: a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br J Cancer 71(4):854–856
Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G (2003) Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res 35(1):12–15
Lissoni P, Paolorossi F, Ardizzoia A, Barni S, Chilelli M, Mancuso M, Tancini G, Conti A, Maestroni GJ (1997) A randomized study of chemotherapy with cisplatin plus etoposide versus chemoendocrine therapy with cisplatin, etoposide and the pineal hormone melatonin as a first-line treatment of advanced non-small cell lung cancer patients in a poor clinical state. J Pineal Res 23(1):15–19
Dillon DC, Easley SE, Asch BB, Cheney RT, Brydon L, Jockers R, Winston JS, Brooks JS, Hurd T, Asch HL (2002) Differential expression of high-affinity melatonin receptors (MT1) in normal and malignant human breast tissue. Am J Clin Pathol 118(3):451–458
Cos S, Fernandez R, Guezmes A, Sanchez-Barcelo EJ (1998) Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res 58:4383–4390
Cos S, Sanchez-Barcelo EJ (2000) Melatonin and mammary pathological growth. Front Neuroendocrinol 21(2):133–170
Grant SG, Melan MA, Latimer JJ, Witt-Enderby PA (2009) Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med 11:e5
Sanchez-Barcelo EJ, Cos S, Mediavilla D, Martinez-Campa C, Gonzalez A, Alonso-Gonzalez C (2005) Melatonin–estrogen interactions in breast cancer. J Pineal Res 38(4):217–222
Jablonska K, Pula B, Zemla A, Owczarek T, Wojnar A, Rys J, Ambicka A, Podhorska-Okolow M, Ugorski M, Dziegiel P (2013) Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res 54(3):334–345
Acknowledgments
We are grateful to the participants of this trial, as well as to the staff at the Dana-Farber Cancer Institute that helped facilitate the trial’s conduct. This study was supported by a grant from the National Cancer Institute (R03 CA123597). The funding source had no role in the design or analysis of the study or in the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W.Y., Giobbie-Hurder, A., Gantman, K. et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat 145, 381–388 (2014). https://doi.org/10.1007/s10549-014-2944-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2944-4