Abstract
To describe which providers provide breast cancer survivorship care, we conducted a longitudinal survey of nonmetastatic breast cancer patients identified by the SEER registries of Los Angeles and Detroit. Multinomial logistic regression examined the adjusted odds of surgeon compared with a medical oncologist follow-up or primary care provider compared with medical oncologist follow-up, adjusting for age, race/ethnicity, insurance, tumor stage, receipt of chemotherapy, endocrine therapy use, and visit to a medical oncologist at the time of diagnosis. Results were weighted to account for sample selection and nonresponse. 844 women had invasive disease and received chemotherapy or endocrine therapy. 65.2 % reported medical oncologists as their main care provider at 4 years, followed by PCP/other physicians (24.3 %) and surgeons (10.5 %). Black women were more likely to receive their follow-up care from surgeons (OR 2.47, 95 % CI 1.16–5.27) or PCP/other physicians (OR 2.62, 95 % CI 1.47–4.65) than medical oncologists. Latinas were more likely to report PCP/other physician follow-up than medical oncologists (OR 2.33, 95 % CI 1.15–4.73). Compared with privately insured women, Medicaid recipients were more likely to report PCP/other physician follow-up (OR 2.52, 95 % CI 1.24–5.15). Women taking endocrine therapy 4 years after diagnosis were less likely to report surgeons or PCP/other physicians as their primary provider of breast cancer follow-up care. Different survivorship care patterns emerge on race/ethnicity and insurance status. Interventions are needed to inform patients and providers on the recommended sources of breast cancer follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current care delivery model for cancer survivorship is unsustainable for three key reasons [1]. First, the number of cancer survivors has increased markedly and will continue to do so due to early detection and treatment advances [2]. Second, the surge in the cancer survivor population coincides with projected shortages of medical oncologists [3, 4] and primary care physicians [5, 6]. Third, follow-up care remains inadequate or fragmented for many cancer survivors [7].
In the case of breast cancer, it is unclear how women should receive follow-up care after treatment. Breast cancer survivors require follow-up to assess for recurrence and late effects of therapy. In particular, women who receive chemotherapy and/or endocrine therapy experience an array of effects, including peripheral neuropathy, osteoporosis, or organ toxicity [8–11]. Breast cancer survivors receive follow-up care in a number of settings. Yet no current guidelines identify the optimal providers of breast cancer care and the optimal time points for transition. Medical oncologists and primary care providers differ in their perspectives and preferences for breast cancer follow-up care [12, 13]. Most survivors receive care from either medical oncologists or primary care physicians; fewer patients receive follow-up from surgical oncologists or other providers [14]. There are divergent preferences from patients [15] and oncologists [16] on the ideal source for breast cancer survivorship care.
A better understanding of the types of providers breast cancer patients see in the survivorship period can inform policies targeted to improve the quality and efficiency of survivorship care. Clinicians and researchers must comprehend the survivorship experience from distinct racial/ethnic groups given the differential mortality rates observed [2] in the absence of disparate chemotherapy treatment [17]. The study objective was to examine patterns of follow-up care by patient, disease, and treatment factors in a large, diverse, population-based sample of breast cancer patients treated with endocrine or chemotherapy. The results fill a knowledge gap of the experience of diverse women seeking long-term breast cancer follow-up.
Patients and methods
Research design
This prospective longitudinal study used rapid case ascertainment methods in partnership with two cancer registries that participate in the surveillance, epidemiology and end-results (SEER) program. The data collection protocol has been summarized previously [18]. Eligible women from Los Angeles County and Metropolitan Detroit were surveyed at two time points. Latinas in Los Angeles and black women in both Detroit and Los Angeles were oversampled. Baseline surveys were mailed to participants between June 2005 and February 2007, which was around 9 months after the initial breast cancer diagnosis. Follow-up surveys were mailed around 4 years after initial diagnosis.
After human subjects’ approval at the University of Michigan and parallel approvals in California and Detroit, cases were reported monthly to SEER registries. We notified physicians by mail of our intent to survey their patients. Next, we mailed patients a packet with a cover letter, a printed survey copy, a statement of study risks and benefits, and a $10 cash gift. To encourage high response rates, we modified the methods specified by Dillman [19]. These included a reminder letter, followed by a second survey to nonrespondents, followed by a follow-up telephone call.
Study participants
Women were eligible to participate if they were between 20 and 79 years of age, diagnosed with ductal carcinoma in situ or invasive nonmetastatic breast cancer (Stages I–III) between June 2005 and February 2007, and reported to the Los Angeles County or Metropolitan Detroit SEER registries. Surveys were administered in both English and Spanish. Asian women were not included due to the second concurrent active study.
Variables
The outcome variable was patient report of the primary provider breast cancer follow-up approximately 4 years after initial diagnosis; this was obtained from the follow-up survey. Women were asked to identify their main provider of breast cancer follow-up care: medical oncologist, surgeon, or primary care physician (PCP) physician. Women were also given the opportunity to name another type of physician. Due to the small number of write-in options, these physicians were lumped into the PCP category.
SEER registries provided age (in years) and cancer stage at diagnosis (I, II, or III) at the baseline survey period. At baseline, patients provided: race/ethnicity (white, black, Latina), receipt of systemic adjuvant chemotherapy (yes/no), insurance coverage (Medicaid, Medicare, private/other insurance, not insured), education (some high school, completed high school, attended or completed college), and whether they consulted a medical oncologist before their initial breast cancer operation. The follow-up patient survey asked women about their use of endocrine therapy for breast cancer; the three response choices were never taken, took in the past but no longer, and current use at the time of survey completion.
Analyses
First, we measured the proportion of women who reported that medical oncologists, surgeons, or primary care/other physicians were their primary provider of breast cancer care approximately 4 years after diagnosis. We then examined differences in the proportion of women who saw medical oncologists versus surgeons or PCPs/other provider types by the covariates listed above. We used multinomial regression to determine the adjusted odds of surgeon versus medical oncologist follow-up as well as the adjusted odds of PCP/other provider versus medical oncologist follow-up. All results were weighted to account for differential probabilities of sample demographics and nonresponse and were conducted in SAS version 10.1.
Results
Of the 1,536 women who completed both baseline and follow-up surveys, 366 were excluded from this analysis due to noninvasive disease and 86 were excluded due to recurrence by the time of the follow-up survey. Because our analysis was focused specifically on women who received systemic therapy, we excluded the 99 women in the sample who did not report at baseline receipt of either chemotherapy or endocrine therapy. 141 women were also excluded because of missing survey data. The final analytic sample consisted of 844 women.
Sample characteristics are presented in Table 1. The sample’s race/ethnicity was white (43.6 %) followed by Latina (40.7 %) and black (15.7 %). The mean (SD) age of respondents was 56.5(11.4) years. The majority of respondents (61 %) reported private insurance coverage, 19.5 % reported Medicare coverage, 11.6 % received Medicaid, and 8.3 % reported no coverage. Most of the sample (69 %) received systemic chemotherapy. 4 years after diagnosis, 45.3 % reported current endocrine therapy use and an additional 28.6 % reported the previous use. Over 90 % of respondents reported a consultation with a medical oncologist before their initial breast cancer operation. Of the 844 women with requisite data for analyses, 65.2 % of women reported medical oncologists as the main provider of survivorship care at 4 years, followed by PCP/other physicians (24.3 %) and surgeons (10.5 %).
Receipt of follow-up by provider type
The multinomial logistic regression model examines two comparative outcomes: receipt of follow-up care by surgeon versus medical oncologists (n = 706) and PCP/other versus medical oncologists (n = 784) (Table 2). Relative to white women, black women were significantly more likely report follow-up with a surgeon (OR 2.47 95 % CI 1.16–5.27) or PCP/other (OR 2.62 95 % CI 1.47–4.65) versus a medical oncologist. Latinas were significantly more likely than white women to be seen by a PCP/other rather than a medical oncologist (OR 2.33 95 % CI 1.15–4.73). Relative to women with private insurance coverage, those with Medicaid coverage were significantly more likely to be seen by a PCP/other than by a medical oncologist for follow-up care (OR 2.52 95 % CI 1.24–5.15). Current endocrine therapy use was associated with medical oncologist follow-up: compared with women who had never taken endocrine therapy, current endocrine therapy users were less likely to see a surgeon (OR 0.35 95 % CI 0.14–0.86) or a PCP/other (OR 0.33 95 % CI 0.17–0.64) than a medical oncologist.
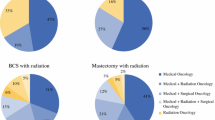
The variables in the multinomial logistic regression model were used to calculate the adjusted proportion of women who reported each type of provider for their follow-up breast cancer care at 4 years (Fig. 1). While the overall majority of women reported medical oncologist follow-up, differences were observed by race/ethnicity: 83 % of white women, 77 % of Latinas, and 65 % of black women reported medical oncologist follow-up (p < 0.001). Whites and Latinas both reported similar rates of surgeon follow-up (6 %), yet 12 % of black women saw surgeons. Black women saw PCP/other physicians more often (23 %) than Latinas (17 %) or white women (11 %).
Discussion
In this diverse sample of women treated with systemic chemotherapy or endocrine therapy for invasive breast cancer, most women reported follow-up care by medical oncologists versus surgeons or PCPs/other provider types. However, significant differences in follow-up care were observed by race/ethnicity and insurance status. In particular, we found black women were significantly less likely than white women to receive breast cancer follow-up care from a medical oncologist versus any other provider type, regardless of receipt of chemotherapy or endocrine therapy. Latinas, too, were less likely to see a medical oncologist versus any other provider type at follow-up, relative to whites. Different patterns of survivorship care emerge for women based on race/ethnicity and insurance status.
Nationally, surgeons provide a very small amount of breast cancer survivorship care relative to medical oncologists and primary care providers [14]. For women who receive systemic and/or endocrine therapy and who do not experience recurrence, it is unclear what aspects of care surgeons would attend to 4 years after diagnosis. Yet black women in our sample reported seeing surgeons at twice the rate of Latinas or white women. These findings point to the need to better understand how breast cancer follow-up care is delivered across diverse settings.
Endocrine therapy after primary breast cancer treatment is recommended for at least 5 years [20]. Recent clinical trials confirm significant survival advantages for tamoxifen therapy extended to 10 years [21, 22]. Yet adherence to 5 years of therapy is low [23–27]. Despite impressive efficacy in reducing recurrence, endocrine therapy is associated with a host of bothersome side effects including, among others, hot flashes, sexual side effects, and arthralgias. Adverse effects often lead women to discontinue therapy [28–32]. Previously reported data from the current study suggest that the subset of women eligible for endocrine therapy was more likely to persist with using therapy when reporting medical oncologist follow-up [27]. It may be the case that women who persist on endocrine therapy follow-up with oncologists for side effect management. Additional studies are needed to understand the processes employed by medical oncologists to support women on endocrine therapy, and disseminate these processes to the range of providers who provide follow-up care in the survivorship setting.
Study limitations
First, we only asked patients to list their primary source of oncology follow-up care, which precludes our ability to measure the presence of shared care models. Patients seen by a team of providers are more likely to receive recommended care [33, 34]. Consequently, a shared care model has recently been proposed as a recommended survivorship care model by both the American Cancer Society and American Society of Clinical Oncology for those patients for whom it is appropriate [11].
Surveys occurred at two time points: approximately nine months and 4 years after diagnosis. Thus, we are not able to determine whether some patients switched their primary follow-up provider between those time points. For example, women may have transitioned from medical oncologist to primary care 3 years after treatment. We lack data on possible reasons for a transition, which in some cases may have been appropriate [35]. Future studies to examine use of different providers in breast cancer survivorship care would benefit from more detailed reasons why patients transition from medical oncologists to other providers.
The primary source of study data derives from patient report. While recall bias is possible, the period in which women were asked to recall specific events is narrow enough to suggest reliable estimates. External data would validate these findings and inform future research focused on care patterns of breast cancer survivors. These limitations are presented alongside a large, diverse population-based sample with high response survey rates at two time points.
Implications
In a population-based sample of diverse early stage breast cancer patients, we found important differences in follow-up care by patient and treatment factors. Amidst a demand surge for cancer care, a substantial number of breast cancer survivors who have not experienced recurrence continue to see their medical oncologists for survivorship care 4 years after initial diagnosis. These results suggest a possible mismatch of needs and resources among breast cancer survivors and available providers. Differences in survivorship care patterns by race/ethnicity require clarification. Specifically, the role of surgical follow up for 12 % of black women is unclear in the absence of disease recurrence.
From the perspective of clinical policy development, there is an urgent need for specialties that treat women with breast cancer to reach consensus on transition plans that are acceptable to both providers and patients. Doing so will increase the likelihood of an efficient, patient-centered cancer care delivery system [1]. Care coordination in the survivorship period can optimize outcomes for breast cancer patients, yet care transitions from oncology to non-oncology practices are difficult for the both patients and providers [36, 37]. Intervention studies have failed to improve outcomes for patients who receive survivorship care plans that are shared across providers [38].
It is important to emphasize the absence of empirical data to develop breast cancer survivorship guidelines. Not all patients need to see a medical oncologist 4 years after treatment [35]. Primary care providers have indicated their willingness to participate earlier in cancer survivorship care [39]. Suggestions to improve their comfort include ready access to oncology specialists, treatment summaries, and access to imaging. A dialog among care providers to breast cancer is needed to clarify transition points and care goals. Educational interventions—targeted to patients and providers—are needed to guide decision making for survivorship care. Our data suggest interventions targeted to specific groups based on race/ethnicity and insurance status may prove useful in responsible resource allocation.
References
Levit L, Balogh E, Nass S, et al (2013) Committee on Improving the Quality of Cancer Care. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. National Academies Press, Washington DC
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30
Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M (2007) Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract 3:79–86
Warren JL, Mariotto AB, Meekins A, Topor M, Brown ML (2008) Current and future utilization of services from medical oncologists. J Clin Oncol 26:3242–3247
Salsberg E, Grover A (2006) Physician workforce shortages: implications and issues for academic health centers and policymakers. Acad Med 81:782–787
Starfield B, Fryer GE Jr (2007) The primary care physician workforce: ethical and policy implications. Ann Farm Med 5:486–491
Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I (2011) Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol 29(10):1280–1289
Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE (2003) Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol 21(22):4184–4193
Carver JR, Shapiro CL, Ng A et al (2007) American society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 25(25):3991–4008
Camp-Sorrell D (2006) Cardiorespiratory effects in cancer survivors. Cancer Nurs 29(2 Suppl):55–59
McCabe MS, Bhatia S, Oeffinger KC et al (2013) American society of clinical oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol 31(5):631–640
Cheung WY, Aziz N, Noone AM et al (2013) Physician preferences and attitudes regarding different models of survivorship care: a comparison of primary care providers and oncologists. J Cancer Surviv 7(3):343–354
Klabunde CN, Han PK, Earle CC et al (2013) Physician roles in the cancer-related follow-up care of cancer survivors. Fam Med 45(7):463–474
Parmeshwar R, Margenthaler JA, Allam E et al (2013) Patient surveillance after initial breast cancer therapy: variation by physician specialty. Am J Surg 206(2):218–222
Mayer DK, Gerstel A, Leak AN, Smith SK (2012) Patient and provider preferences for survivorship care plans. J Oncol Pract 8(4):e80–e86
Potosky AL, Han PK, Rowland J et al (2011) Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med 26(12):1403–1410
Griggs JJ, Hawley ST, Graff JJ et al (2012) Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol 30(25):3058–3064
Janz NK, Mujahid MS, Hawley ST et al (2009) Racial/ethnic differences in quality of life after diagnosis of breast cancer. J Cancer Surviv 3(4):212–222
Dillman DA (2007) Mail Internet Surveys: the tailored design method, 2nd edn. Wiley, New York
Burstein HJ, Prestrud AA, Seidenfeld J et al (2010) American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with endocrine receptor-positive breast cancer. J Clin Oncol 28(23):3784–3796
Davies C, Hongchao P, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816
Jin H, Tu D, Zhao N et al (2012) Longer-term outcomes of Letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 30(7):718–721
Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G (2012) Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol 36(2):181–187
Hershman DL, Shao T, Kushi LH et al (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537
Hershman DL, Kushi LH, Shao T et al (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128
Partridge AH, Lafountain A, Mayer E et al (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562
Friese CR, Pini TM, Abrahamse PH et al (2013) Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat 138(3):931–939
Atkins L, Fallowfield L (2006) Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 42:2271–2276
Demissie S, Silliman RA, Lash TL (2001) Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19:322–328
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA (2004) Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Oncol Pract 8(6):e149–e157
Aiello Bowles EJ, Boudreau DM, Chubak J et al (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8(6):e149–e157
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM (2007) Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care 45:431–439
Snyder CF, Frick KD, Kantsiper ME et al (2009) Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol 27(7):1054–1061
Snyder CF, Frick KD, Peairs KS et al (2009) Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med 24(4):469–474
Earle CC, Ganz PA (2012) Cancer survivorship care: don’t let the perfect be the enemy of the good. J Clin Oncol 20(30):3764–3768
Hewitt M, Greenfield S, Stovall E (2005) From cancer patient to cancer survivor: Lost in transition. National Academies Press, Washington
Kantsiper M, McDonald EL, Geller G et al (2009) Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med 24(Suppl 2):459–466
Grunfeld E, Julian JA, Pond G et al (2011) Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol 29(36):4755–4762
Del Guidice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S (2009) Primary care physicians’ views of routine follow-up care of cancer survivors. J Clin Oncol 27:3338–3345
Acknowledgments
This study was supported by Grants R01 CA109696 and R01 CA088370 from the National Cancer Institute (NCI) to the University of Michigan. Dr. Friese was supported by a pathway to independence award from the National Institute for Nursing Research (R00NR01570). Dr. Jagsi was supported by a mentored research scholar grant from the American Cancer Society (MRSG-09-145-01). Dr. Katz was supported by an established investigator award in cancer prevention, control, behavioral, and population sciences research from the NCI (K05CA111340). The collection of LA County cancer incidence data used was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s SEER program under contract N01-PC-35139 awarded to the University of Southern California, contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The collection of metropolitan Detroit cancer incidence data was supported by the NCI SEER Program contract N01-PC-35145.
Conflict of interest
The authors declare they have no conflict of interest.
Ethical standards
The study complied with the ethical standards set forth for human subjects by the University of Michigan, Detroit, and California sites.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friese, C.R., Martinez, K.A., Abrahamse, P. et al. Providers of follow-up care in a population-based sample of breast cancer survivors. Breast Cancer Res Treat 144, 179–184 (2014). https://doi.org/10.1007/s10549-014-2851-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2851-8