Abstract
Fulvestrant, which degrades ER, is used after AI failure in metastatic breast cancer but resistance develops quickly. We hypothesized that using everolimus to inhibit mTOR, a key signaling pathway in endocrine resistance, may delay fulvestrant resistance in patients and thus improve its efficacy. We conducted a phase II trial of combined fulvestrant and everolimus in postmenopausal women with disease progression or relapse after an AI. Primary endpoint was time to progression (TTP) and secondary endpoints included objective response rate, clinical benefit rate (CBR), safety, and biomarker correlates. Tumor blocks were collected and biopsy of accessible tumor was done for future biomarker analysis. Of 33 patients enrolled two were ruled ineligible after enrollment and were excluded from study analysis, for a total of 31 evaluable patients. Median age was 54 years (range 45–85). Prior therapy included tamoxifen (81 %), chemotherapy (71 %), with 26 % of patients having received 3 or more endocrine agents. Median TTP was 7.4 months (95 % CI 1.9–12.1) with an objective response rate of 13 % and CBR of 49 %. Of particular note, 32 % of patients exhibited de novo resistance to study treatment with disease progression as their best response. Most common adverse events (AEs) were elevated AST (87 %) and ALT (77 %), anemia (74 %), hyperglycemia (71 %), and hypercholesterolemia (68 %). Prominent clinical toxicities were mucositis (58 %), weight loss (48 %), and rash (42 %). Most AEs were grade 1 or 2 and largely reversible with infrequent need for everolimus dose reduction. To conclude, everolimus plus fulvestrant is effective after AI failure in heavily pretreated metastatic ER-positive breast cancer and has manageable toxicity. Further study of this combination is warranted in randomized studies. Since not all patients experience benefit, and in view of potential toxicities, biomarker examination is critical to help select patients most likely to benefit from this strategy in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine therapy is indispensable in the treatment of women with metastatic ER-positive breast cancer and can prevent disease progression for extended periods of time with minimal toxicity and cost while preserving quality of life. New-generation AIs, which lower serum estrogen levels and cause tumor estrogen deprivation, have largely replaced tamoxifen as the standard first-line therapy for postmenopausal women with metastatic ER-positive disease [1–3]. Fulvestrant, an antiestrogen which degrades ER and, unlike tamoxifen, has no known agonist effect [4], has emerged as an additional treatment option for metastatic disease, particularly after failure of AI therapy [5]. Despite the benefits and recent progress made in expanding endocrine therapy options, however, therapeutic resistance remains a major obstacle. While some patients have de novo endocrine-resistant disease, others experience only limited benefit, and ultimately all patients experience disease progression necessitating the use of chemotherapy [6]. Developing strategies to overcome clinical endocrine resistance is of paramount importance to improving treatment outcomes in patients with this disease.
Growing preclinical and clinical evidence suggests that molecular crosstalk between ER and other critical survival and growth signaling pathways in breast cancer may contribute to endocrine resistance and treatment failure in patients [6]. This improvement in understanding ER biology and its relevance to endocrine resistance, coupled with the recent availability of novel pathway inhibitors, has contributed to the development of a variety of clinical trial strategies combining endocrine and other targeted agents in order to delay hormone therapy resistance in patients [7].
One of the most critical signaling pathways that may emerge after prolonged estrogen deprivation in ER-positive breast cancer is the PI3K/AKT/mTOR survival pathway [8, 9] and may, therefore, be an important feature of progressive disease biology after AI failure [10]. Preclinical models of ER-positive breast cancer have shown that inhibition of PI3K/AKT/mTOR signaling can restore endocrine sensitivity and prevent tumor growth [11–13]. The biologic relevance of PI3K/AKT/mTOR signaling in preclinical endocrine resistance models is further supported by its clinical relevance, with the observation of increased pathway aberrations in ER-positive breast cancer compared to other breast cancer subtypes [14], supporting a potential central role in clinical endocrine resistance [13, 15].
Based on this rationale, we conducted a phase II clinical trial combining the oral mTOR inhibitor everolimus with fulvestrant in patients with metastatic ER-positive breast cancer after AI failure. In addition to clinical assessment of treatment efficacy and toxicity, we collected tumor tissue where available and patients with accessible tumor were offered a research biopsy for future biomarker analysis.
Methods
Patients
Postmenopausal patients were required to have metastatic ER-positive breast cancer, measurable and/or evaluable disease, and disease progression or relapse on an AI within 6 months prior to enrollment. Patients were required to have an ECOG performance status of 0–2 and adequate baseline renal and hepatic function, defined as serum creatinine ≤1.5× upper limit of normal (ULN), serum bilirubin ≤1.5× ULN, and ALT/AST ≤2.5× ULN; adequate bone marrow function, defined as an absolute neutrophil count ≥1.5 × 109/l, platelet count >100,000/ul, and hemoglobin >9 gm/dl; and an International Normalized Ratio of <1.3. Key exclusion criteria were rapidly progressive disease requiring chemotherapy and known brain or leptomeningeal metastases. The study was conducted at the University of Kentucky Markey Cancer Center and approved by the University Of Kentucky Institutional Review Board. All patients signed informed consent.
Study design and agent administration
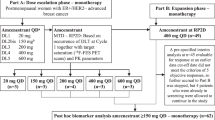
This was a single-institution phase II study of combined fulvestrant and everolimus after AI failure, starting therapy concomitantly on day 1 of enrollment. A core tumor biopsy was offered on study entry if disease was accessible (from primary tumor or skin) and preexisting tumor blocks were collected when available. Fulvestrant was administered intramuscularly (in the gluteus maximus) in a loading dose schedule as follows: 500 mg in two divided doses—one on each side on day 1, then 250 mg on day 14, and then 250 mg on day 28 and every 4 weeks ± 3 days thereafter. Everolimus was administered initially at a dose of 5 mg daily in the first 5-patient cohort for the first month of treatment and then increased to 10 mg PO daily after that. If two or more out of this initial cohort develop grade III toxicity or greater, then a second 5-patient cohort was planned to be recruited at the 5 mg daily dose level and the same toxicity rule followed. If treatment was tolerated based on this toxicity rule, then subsequent patients would start at 10 mg dose level. Since the frequency of grade-3 toxicity in the first 5-patient cohort was lower than that defined in the above rule, all subsequent patients received everolimus at the full starting dose level of 10 mg PO daily. Clinical evaluation for disease progression and tolerability of the regimen was evaluated every 2 weeks for the first two visits and every 4 weeks thereafter in outpatient clinic visits. In addition to routine chemistry and blood counts, fasting lipid profile and glucose were obtained every 2 months on study. After 2 months of enrollment, patients underwent their first formal radiologic disease assessment using RECIST criteria and imaging was done every 2 months thereafter and when indicated by symptoms suggestive of progressive disease (PD). Patients who experienced PD on protocol were taken off study and were required to have a safety follow-up 4 weeks after treatment discontinuation.
Biopsies
Where applicable, breast tumor tissue was obtained by core biopsy using a Bard Max Core Biopsy Instrument (#MC1410). The outside needle was 14-gauge (2.1 mm), with a smaller inside cutting needle to obtain the biopsy. The length of the cutting needle penetration was 22 mm and the total length of the needle was 10 cm. Multiple core biopsy samples of approximately 2 mm × 10–20 mm were divided in 10 % formalin or immediately frozen in liquid nitrogen.
Statistical considerations
This was a single-stage phase II trial of the combination of fulvestrant and everolimus with a primary endpoint of time to progression (TTP) from enrollment. Secondary endpoints were the assessment of response rates per RECIST criteria, clinical benefit rate (CR+PR+SD of 24 weeks or more), toxicity assessment, and exploratory biomarker correlation with treatment benefit. A randomized trial of fulvestrant versus exemestane after non-steroidal AI failure in metastatic breast cancer (EFECT trial) served as the historical control for single-agent fulvestrant effect [5], with a reported median TTP on fulvestrant of 3.7 months. We hypothesized that median TTP in our trial will increase from 3.7 months for the historical fulvestrant-only control to 7.0 months on the combination of fulvestrant and everolimus in the current trial. A sample of 40 evaluable patients was calculated to show the increase in TTP with 80 % power and 5 % significance level based on a two-sided test of differences in survival times between historical controls and treated group. TTP was defined as time from study entry to disease progression or death, whichever occurs first. For patients without disease progression, the outcome was censored at the time of last assessment. Overall survival (OS) was defined as time from study entry until death or was censored at the time of last follow-up. Median TTP and OS were estimated using the Kaplan–Meier product limit survival curve. 95 % confidence intervals (CI) were provided for response and survival data. Wilcoxon signed-rank test was used to compare the median values of lipid profile parameters. Sample size calculations were conducted using NCSS (number cruncher statistical systems) software (www.ncss.com) and adverse events (AEs) were recorded using the NCI CTCAE v3.0 and were summarized by grade and overall frequency. Analyses were completed using SAS 9.3.
Results
Patients and disease characteristics
A total of 33 patients were enrolled on trial between March 2008 and October 2012; short of the planned accrual target, since FDA approval of everolimus for the same patient population in July 2012 lead to a significant drop in accrual. Of the 33 patients enrolled, two were ruled ineligible immediately after enrollment and were excluded from further analysis (one because of elevated creatinine outside the institutional reference range and one who required immediate palliative radiation to painful spine metastasis). Median patient age was 54 years with a range of 45–85 years (Table 1). All tumors were ER-positive and the majority were PgR-positive as well (84 %). Two patients had confirmed HER2-positive disease, one with de novo metastasis and the other one upon biopsy of metastatic disease in the liver at the time of relapse. The most common sites of metastasis were bone (84 %), liver (62 %), and lung (55 %), with 55 % of patients having 3 or more sites of metastatic disease (Table 1). Twenty-two patients (71 %) had received chemotherapy previously, 19 of them (61 %) in the adjuvant setting. Upon study entry, 74 % of the patients were receiving the AI as treatment for metastatic disease and 26 % as adjuvant therapy. Overall, patients were relatively heavily pretreated with 26 % receiving protocol treatment as their 4th-line endocrine therapy. Of particular note, 32 % of patients were classified as having AI-resistant disease (defined as relapse within the first 3 years of adjuvant AI or disease progression within 6 months of AI therapy for metastatic disease).
Efficacy analysis
Out of 31 evaluable patients, 29 (94 %) have discontinued therapy at the time of this report. Most common reasons for treatment discontinuation were disease progression (71 %), study-related toxicity (10 %), unrelated intercurrent illness (6 %), and patient choice (6 %), with 2 patients currently remaining on treatment (32 and 16 months, respectively). TTP is 7.4 months (95 % CI 1.9–12.1) (Fig. 1). The best response data using RECIST criteria are summarized in Table 2. The most frequent response observed was stable disease (42 %). There was one patient with liver-only metastatic disease who experienced complete response (CR) and remains on study treatment 32 months after enrollment. Partial response (PR) was observed in 3 patients (10 %) with an additional 2 patients with SD experiencing 26 and 28 % reduction in target disease measurement, respectively, just short of a PR. One patient (85 year old) who discontinued treatment in the first month because of grade-3 pneumonia and decline in her performance status experienced a dramatic disappearance of chest wall skin nodules and an 18 % reduction in her liver metastasis on imaging studies done one month after treatment. Overall, clinical benefit rate was 49 %. Interestingly, one-third of the patients exhibited de novo resistance to treatment as judged by first radiologic disease assessment at 8 weeks, and 2 additional patients experienced progression on the second disease assessment at 16 weeks. There were no discernible clinical variables that predicted duration of treatment benefit, but the majority of long-term responders were previously AI-sensitive as illustrated by individual patient treatment graph (Fig. 2).
Graph of individual patient duration of treatment with everolimus and fulvestrant in months. Patients with AI-sensitive disease are shown in blue and those with AI-resistant disease in red. The asterisks indicate patients who were censored at the indicated time points and the arrow indicates patients who continue on treatment at the time of this publication
At the time of this report, 22 patients have expired, none of them while on study treatment, with a median overall survival (OS) of 24.0 months (95 % CI 18.3–28.7) (Fig. 3).
Safety
All patients experienced some toxicity, summarized in Table 3. The most common dose-limiting toxicity was mucositis, with 2 patients requiring everolimus dose interruption and then reduction in the first month of treatment, with both these patients experiencing disease progression on first disease assessment at 2 months. A third patient discontinued therapy in the first month of treatment because of grade-3 mucositis and diarrhea despite dose interruption and reduction. One additional patient experienced intermittently recurrent mucositis over a year after enrollment necessitating everolimus dose reduction and her disease subsequently progressed after 4 months of the dose reduction. Pneumonia occurred in 5 patients, requiring treatment discontinuation in one, while the others recovered after appropriate antibiotic treatment and remained on protocol. The relationship of pneumonia to study treatment is unclear but was most often judged to be community-acquired. Grade-3 toxicities were infrequent, with one grade-4 toxicity recorded (hypokalemia) and no patients expired while on study. Overall, the majority of toxicities were grade 1 and grade 2 and were reversible with everolimus interruption or dose reduction.
Of particular note was the frequent development of metabolic changes on treatment, including hyperglycemia (71 %) and hypercholesterolemia (68 %). To further analyze the impact of everolimus on serum lipid profiles, we compared fasting lipids at baseline and after 2 months of treatment (Table 4). There was a significant and consistent increase in all lipid profile parameters (p < 0.001) which persisted throughout follow-up bimonthly testing while on study treatment (data not shown).
Discussion
Our study shows promising activity of combining everolimus with fulvestrant after AI failure, confirming the central role of the PI3K/AKT/mTOR pathway in endocrine resistance [13] and consistent with recent clinical trials results of combining everolimus with other endocrine agents in endocrine-resistant disease [16, 17]. To our knowledge, this is the first combination-targeted strategy with fulvestrant to show promising benefit in the AI-resistant disease setting. Several other clinical trials combining fulvestrant with other signaling pathway inhibitors after AI failure were conducted, but the results were largely disappointing, including combinations with angiogenesis inhibitors [18, 19], insulin growth factor-1 receptor (IGF1R) antibodies [20], and inhibitors of EGFR/HER2 [21, 22]. Our findings strongly support the role of the PI3K/AKT/mTOR signaling as a common pan-endocrine resistance pathway, across the different endocrine therapy strategies tested to date.
Fulvestrant in particular, and despite its superiority in preclinical models of ER-positive breast cancer [23], clinical trials somewhat disappointingly showed more modest results in comparison to either tamoxifen [24] or aromatase inhibitors [5] [25, 26]. Because of the established superiority of AIs over tamoxifen in the first-line metastatic setting [1, 2], fulvestrant was incorporated after AI failure in the second-line metastatic disease setting, with a relatively modest median TTP of 3.7 months [5]. Subsequent research into improving clinical efficacy of fulvestrant focused on three separate approaches: the use of a higher dose, combination with AI therapy to counteract competition from estrogen on ER inhibition, and combinatorial strategies with various inhibitors of pathways implicated in endocrine resistance. At the time this study was conducted, the FDA-approved dose of fulvestrant was the loading dose regimen and this was the dose used in our study for comparison with the reported TTP from the EFECT trial [5]. In a subsequent study, high-dose fulvestrant was found to have a superior progression-free survival compared to the loading dose regimen, although the patient population was not restricted to those experiencing AI failure, and in that subset of patients who received an AI as their last treatment, benefit of high-dose fulvestrant was less pronounced [27].
The second strategy to improve fulvestrant efficacy was to combine it with an AI in the second-line metastatic setting after initial AI therapy failure in order to enhance ER blockade, but this strategy was no better than the steroidal AI exemestane or fulvestrant alone, suggesting that the strategy of combined endocrine therapy after AI failure was not a beneficial approach [28]. Interestingly, in the first-line setting, the combination of fulvestrant and an AI was more effective than an AI alone, but there was no fulvestrant-only control arm in that study [29] and another study failed to show superiority of combined fulvestrant and AI versus AI alone [30]. In all these aforementioned endocrine combination studies, the loading dose fulvestrant regimen was used and no combination endocrine studies have been conducted to date with the currently FDA-approved high-dose fulvestrant regimen. It is not currently known whether using the higher dose instead of loading dose fulvestrant strategy will offer additional benefit in the second-line setting in future combination trials with PI3K/mTOR inhibitors.
The efficacy of the combination of fulvestrant and everolimus in our strategy may well be restricted to the second-line setting after initial AI failure based on the biologic rationale of the relevance of PI3K/AKT/mTOR signaling in endocrine-resistant disease. An earlier study combining another mTOR inhibitor, oral temsirulimus, with letrozole in first-line metastatic disease resulted in no additional benefit for the combination compared to letrozole alone [31]. It is possible that the negative results in first line maybe related to the particular combination of agents used, although patients on temsirulimus did experience the classic toxicities ascribed to this class of drugs. Whether the strategy of combining everolimus or newer inhibitors of PI3K/AKT/mTOR with endocrine therapy retains superior activity in the first-line metastatic setting remains to be seen in future randomized studies.
Interestingly, while the combination of everolimus and fulvestrant appears to be active in our study, it is clear that at least a third of patients did not derive any benefit in this unselected population. Certainly, better selection of patients for future targeted therapy clinical trials is needed. This is particularly important in view of the potential toxicities of everolimus which, even though less than would be expected from using chemotherapy, are still greater than endocrine therapy alone. Short-term toxicity, particularly mucositis, can be dose limiting and may lead to treatment discontinuation or dose reductions which, despite the small numbers, appears to be consistently associated with disease progression in this study.
Of critical importance are the metabolic changes observed with relative consistency on everolimus, including hyperglycemia and dyslipidemia (also see Busaidy et al. [32]), which carry significant implications for future adjuvant trials and has potential impact on long-term risk of cardiovascular disease. Monitoring and appropriate treatment of lipid and glucose changes, if indicated, would be an important consideration in balancing potential benefit with toxicity in the early disease setting.
Because of these potential toxicities of mTOR inhibitors and our observation that about a third of the patients derived no benefit from this strategy, it is critical to identify biomarkers that correlate with treatment benefit and thus allow for better future patient selection. This will not only spare toxicity for those unlikely to respond, but will allow them to participate in more suitable clinical trials for their specific disease subsets. Through target enrichment in future trial designs, statistical power can be achieved with fewer patients to answer a specific study question. Biomarker analysis from this trial is ongoing and may help shed light onto predictors of benefit from the use of mTOR inhibitors with fulvestrant.
In conclusion, everolimus improves fulvestrant efficacy in patients with metastatic ER-positive breast cancer after failure of AI therapy and these findings are consistent with a central role for the PI3K/mTOR pathway across commonly used endocrine therapy strategies. Future randomized studies of combined everolimus and fulvestrant are needed to confirm these findings in the endocrine-resistant disease setting. In addition, it may be worthwhile examining whether use of everolimus in combination with fulvestrant, or other endocrine agents, may be of benefit in delaying the emergence of resistance in earlier settings or in endocrine-sensitive disease. Regardless of which setting this strategy is used, it is critical to identify potential molecular predictors of benefit from mTOR inhibition, for better selection of patients and to spare patients who are unlikely to benefit the potential toxicities associated with this treatment strategy.
References
Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92(9):2247–2258. doi:10.1002/1097-0142(20011101)92:9<2247:AID-CNCR1570>3.0.CO;2-Y
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19(10):2596–2606
Mauri D, Pavlidis N, Polyzos NP, Ioannidis JPA (2006) Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 98(18):1285–1291. doi:10.1093/jnci/djj357
Osborne C, Wakeling A, Nicholson R (2004) Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 90(Suppl 1):S2–S6
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26(10):1664–1670. doi:10.1200/JCO.2007.13.5822
Massarweh S, Schiff R (2006) Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer 13(Supplement 1):S15–S24. doi:10.1677/erc.1.01273
Leary A, Sirohi B, Johnston S (2007) Clinical trials update: endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res 9(5):112
Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A (2007) Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res 13(9):2751–2757. doi:10.1158/1078-0432.ccr-06-2466
Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J Jr, Berstein L, Yue W (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Supplement 1):S61–S73. doi:10.1677/erc.1.01018
Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ (2001) Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res 61(16):5985–5991
Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, DeGraffenried LA (2007) Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol 18(8):1323–1328. doi:10.1093/annonc/mdm170
Treeck O, Wackwitz B, Haus U, Ortmann O (2006) Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol 102(2):292–299
Miller TW, Balko JM, Arteaga CL (2011) Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol 29(33):4452–4461. doi:10.1200/jco.2010.34.4879
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo W-L, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung M-C, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091. doi:10.1158/0008-5472.can-07-6854
Bachelot T (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO Study. J Clin Oncol 30:2718–2724
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Hyams DM, Chan A, de Oliveira C, Snyder R, Vinholes J, Audeh MW, Alencar VM, Lombard J, Mookerjee B, Xu J, Brown K, Klein P (2013) Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a randomized Phase II study. Invest New Drugs 31(5):1345–1354. doi:10.1007/s10637-013-9991-2
Tan WW, Dueck AC, Flynn P, Steen P, Anderson D, Rowland K, Northfelt D, Perez EA (2013) N0539 phase II trial of fulvestrant and bevacizumab in patients with metastatic breast cancer previously treated with an aromatase inhibitor: a North Central Cancer Treatment Group (now Alliance) trial. Ann Oncol 24(10):2548–2554
Robertson JF, Ferrero JM, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I, Loh E, Gansert JL, Kaufman PA (2013) Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 14(3):228–235. doi:10.1016/S1470-2045(13)70026-3
Burstein HJ, Barry WT, Cirrincione C, Chew HK, Tolaney S, Lake D, Pluard T, Blackwell K, Winer EP, Hudis CA (2010) Fulvestrant with or without lapatinib as therapy for hormone receptor positive advanced breast cancer: a double-blinded, placebo-controlled, randomized phase III study. Cancer Res 70(24 Suppl 2):Abstr nr PD-05-01. doi:10.1158/0008-5472.SABCS10-PD05-01
Carlson RW, O’Neill A, Vidaurre T, Gomez HL, Badve SS, Sledge GW (2012) A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat 133(3):1049–1056. doi:10.1007/s10549-012-1997-5
Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI (1995) Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst 87(10):746–750
Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, Watanabe T, Morris C, Webster A, Dimery I, Osborne CK (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 22(9):1605–1613. doi:10.1200/JCO.2004.02.112
Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, Vergote I, Erikstein B, Webster A, Morris C (2002) Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 20(16):3396–3403
Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, Gertler SZ, May JT, Burton G, Dimery I, Webster A, Morris C, Elledge R, Buzdar A (2002) Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20(16):3386–3395
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JPO, Sapunar F, Martin M (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg With fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28(30):4594–4600. doi:10.1200/jco.2010.28.8415
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, Im YH, Braybrooke JP, Brunt AM, Cheung KL, Jyothirmayi R, Robinson A, Wardley AM, Wheatley D, Howell A, Coombes G, Sergenson N, Sin HJ, Folkerd E, Dowsett M, Bliss JM (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998. doi:10.1016/S1470-2045(13)70322-X
Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, Lew DL, Hayes DF, Gralow JR, Livingston RB, Hortobagyi GN (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367(5):435–444. doi:10.1056/NEJMoa1201622
Bergh J, Jönsson P-E, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, Lindemann JPO, Wiklund F, Henriksson R (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30(16):1919–1925. doi:10.1200/jco.2011.38.1095
Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, Sun Y, Neskovic-Konstantinovic Z, Guimaraes RC, Fumoleau P, Chan A, Hachemi S, Strahs A, Cincotta M, Berkenblit A, Krygowski M, Kang LL, Moore L, Hayes DF (2012) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. doi:10.1200/jco.2011.38.3331
Busaidy NL, Farooki A, Dowlati A, Perentesis JP, Dancey JE, Doyle LA, Brell JM, Siu LL (2012) Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 30(23):2919–2928. doi:10.1200/JCO.2011.39.7356
Acknowledgments
This work was supported by a grant from Novartis pharmaceuticals (S. M.). Presented in part at the 35th San Antonio Breast Cancer Symposium in 2012 (Cancer Res, December 15, 2012; 72(24 Supplement): P2-14-05, and at the 2013 American Society of Clinical Oncology Meeting, Chicago (J Clin Oncol 31, 2013, suppl; abstr 541). Registered under ClinicalTrials.gov number NCT00570921 and Investigational New Drug Application (IND) #79,103 (IND holder, S. M.).
Conflict of interests
S.M. receives research funding from Novartis and Onyx–Bayer, and honoraria/consultation fees from Novartis. All remaining authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massarweh, S., Romond, E., Black, E.P. et al. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)-positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res Treat 143, 325–332 (2014). https://doi.org/10.1007/s10549-013-2810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2810-9