Abstract
Infiltrating lobular carcinoma (ILC) of the breast is associated with greater oestrogen receptor expression and poorer response to neoadjuvant chemotherapy, when compared to infiltrating ductal carcinoma (IDC). In order to compare the pathological complete response rate (pCR) and breast conserving surgery (BCS) in patients with ILC versus IDC treated with neoadjuvant chemotherapy, we performed a systematic review and meta-analysis of all published studies. A search of PubMed, EMBASE, the Web of Science, SCOPUS and the Cochrane Central Register of Controlled Trials was performed to identify studies that investigated pCR, clinical response and BCS in patients with ILC that were treated with neoadjuvant chemotherapy. Random-effect models were adopted to estimate the summary odds ratio (OR), and the publication bias was evaluated using a funnel plot and Egger’s regression asymmetry test. Seventeen studies were included (one randomized controlled trial, three prospective series and 13 retrospective trials), for a total of 12,645 IDCs and 1,764 ILCs to be compared. Ductal carcinoma of the breast was associated with a better pCR (from 5.9 to 16.7 %; OR = 3.1, 95 % CI 2.48–3.87, P < 0.00001) and rate of BCS (from 35.4 to 54.8 %; OR = 2.1, 95 % CI 1.8–2.45, P < 0.00001) compared to ILC. The overall pCR rates and BCS decreased in the ILCs compared with IDC when treated with neoadjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infiltrating lobular carcinoma (ILC) is the second most common subtype of invasive breast cancer (BC), accounting for approximately 5–10 % of all invasive tumours. Infiltrating lobular carcinoma offers an exclusive clinical/radiological presentation and pathological appearance, and presents with different features, compared to infiltrating ductal carcinoma (IDC). For example, ILC is always an oestrogen-receptor positive tumour (ER+) with a high frequency of multicentricity and bilaterality. It is more commonly associated with older age, larger diameter and well-differentiated morphology. While older series report similar prognoses for ILC and IDC, more recent reports suggest that the outcomes (at least in the short-term) may be more favourable for lobular cancers, with improvement over time [1–5].
Neoadjuvant chemotherapy is the treatment that precedes the locoregional treatment of BC, and is indicated for large operable tumours that are not amenable to conservative surgery, or for locally advanced inoperable BCs to improve locoregional control. It usually consists of polychemotherapy, with anthracycline plus or minus a taxane, for at least 3–6 months. After neoadjuvant polychemotherapy, a complete disappearance of tumour cells in the breast and lymph nodes (a pathological complete response; pCR) is achieved in a variable rate of patients, ranging from <5–10 % (for ER+ tumours) to 30–40 % for triple negative and HER2−positive BC [6–8]. Obtaining a pCR is prognostic of the best survival in BC, as in other oncological diseases such as rectal cancer and bladder cancer. In a meta-analysis of 12 neoadjuvant randomized controlled trials in BC, pCR was associated with a better outcome [hazard ratio (HR) = 0.36, P < 0.001] and event-free survival (HR = 0.48, P < 0.001) [9]. In that meta-analysis, the pCR was significantly associated with G3 tumours, ductal histology, ER and progesterone (PgR) negative (other than HER2+/ER−) and triple negative disease.
Current guidelines recommend the use of neoadjuvant chemotherapy with anthracycline based combinations, even in ER+/HER2–BC, and even if few patients are expected to reach a pCR. In these cases, tumour shrinkage may allow some patients to receive conservative surgery and some patients with unresectable disease to undergo surgery. Generally, those who are medically unfit for or refuse chemotherapy are treated with neoadjuvant endocrine therapy. In ER+ disease, the rates of clinical RR and pCR were similar with chemotherapy and endocrine therapy (anastrozole or exemestane) in one randomized trial [10]. Historically, poor activity with neoadjuvant chemotherapy was described in ILC histology with pCR rates in about 5 % of the cases [5]. It seems that the molecular characteristics, more so than pure lobular histology, are predictors of pCR in cases of ILC [11].
Here, we present a meta-analysis evaluating the association of lobular histology with pathological response to neoadjuvant chemotherapy in BC. The primary aim of this study was to evaluate the rate of pCR in patients with operable or locally advanced ILC, treated with neoadjuvant chemotherapy, in comparison to IDC.
Methods
Search strategy and selection of studies
PubMed, the Web of Science, EMBASE, SCOPUS and the Cochrane Register of Controlled Trials (CENTRAL) were searched for studies evaluating the correlation of lobular histology with pCR after neoadjuvant chemotherapy in BC, from 1990 to August 25th, 2013. We used the medical subject heading terms ‘carcinoma, lobular’ and limited the results to human studies. In addition, we used the entry terms ‘neo-adjuvant or preoperative or primary or chemotherapy or pathologic complete response’ to identify additional studies. Eligibility criteria included the proportion of pCRs in ILC compared to IDC, availability of clinical overall response rate (RR) other than partial and complete RR, rate of breast conservation and publication in English. Studies evaluating endocrine therapy or targeted therapies were excluded from this analysis. In addition, the reference lists of the retrieved articles were checked to identify additional relevant publications. The ‘Related Articles’ function was also used to improve the search. Study selection was based on the association of ILC histology with the pCR rate. The study selection, data extraction and data entry were performed by 2 authors independently (FP and SB), and discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigator (SB).
Data extraction
The following information was extracted from each article: (1) basic information, including the year of publication and the first author’s name; (2) study information, including pCR definition, sample size, study design, number of ILC and IDC patients, and biological characteristics of ILC/IDC tumours; (3) treatment information, including neoadjuvant schedules and number of cycles and (4) outcomes of interest, such as the percentage or number of pCRs in the ILC and IDC population, overall clinical RR (ORR) with clinical partial and complete responses (cPR and cCR) if available, and rate or number of breast conserving therapies (BCT) in the ILC and IDC population. If lobular histology was a poor prognostic factor for pCR, then the BCT or outcome in the multivariate analysis was also recorded in any trial.
Statistical analysis
The pCR was the primary outcome measure, and the ORR, cPR, cCR and BCT were the secondary endpoints. The pCR and other comparisons in the ILC and IDC subgroups were calculated using the method for dichotomous data [assessment of odds ratio (OR); 95 % CI]. Cochran’s Q-test and I 2 statistics were used to assess heterogeneity between the studies, and the random-effects model was used for the analysis. A meta-analysis was performed according to the DerSimonian and Laird method.
Finally, potential publication biases were evaluated using funnel plots for the pCR analysis, which assessed the relative symmetry of the individual study estimates around the overall estimate, followed by the Begg’s and Egger’s tests. A two-tailed P value < 0.05 without adjustment for multiplicity was considered to be statistically significant. The leave-one-out procedure was also performed for the primary endpoint analysis. The ‘fail-safe N’ was calculated, which is defined as the number of additional ‘negative’ studies (studies in which the intervention effect was zero) required to increase the P value for the meta-analysis to above 0.05. A two-tailed P value < 0.05 was considered to be statistically significant, and the results of the meta-analysis were reported as classic forest plots (for the pCR meta-analysis).
All statistical analyses were performed using Review Manager 5.1 (RevMan [computer programme] version 5.1; Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2008) and Comprehensive Meta-Analysis software (version 2.2.064; July 27, 2011).
Results
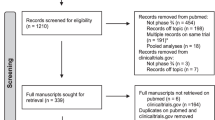
A total of 4,207 references were identified through the five electronic database searches, and a summary of the study selection process is summarized in Fig. 1. After the exclusion of duplicate references and applying the selection criteria, 17 studies remained for assessment [8, 11–26]. A manual search of the reference lists did not identify any additional relevant studies, and full articles were obtained and further evaluated. Of the 17 studies included for final analysis in the present systematic review, one study was a randomized controlled trial, three were prospective series, and the remainder was from retrospective trials as summarized in Table 1. In these 17 studies, a total of 12,645 IDCs and 1,764 ILCs were compared. The definition of pCR was very similar in all studies: the absence of any invasive BC cells in the primary tumour and lymph nodes in n = 13 trials, only in the primary tumour in n = 2 trials, an absence of invasive cells without site specification in n = 1 trial, and the definition was not reported in n = 2 trials. Neoadjuvant regimens consisted invariably in anthracycline-based chemotherapy, plus or minus a taxane, for a minimum duration of three cycles to a maximum of about 6 months overall.
Primary endpoint: pCR
Overall pCR data were available in all of the studies. Pathological complete response ranged from 0 to 38.6 % in the ILCs, and from 6 to 46.2 % in the IDCs. The pooled pCR was 5.9 % (95 % CI 3.6–9.4 %) for ILC and 16.7 (95 % CI 13.5–20.5) for IDC, according to the random effects model. The pooled OR was 3.1 (95 % CI 2.48–3.87, P < 0.00001; Fig. 2) according to the random effects model with low heterogeneity (I 2 = 0 %, P for heterogeneity 0.48).
Secondary endpoints: ORR, cPR, cCR, and breast conservation
The ORR was available in n = 4 studies, and ranged from 26 to 75 % for the ILC and from 58 to 85 % for the IDC. Clinical PRs were present in only 2 studies, and ranged from 26.3 to 35 % for the ILC and from 41 to 49.1 % for the IDC. Clinical CRs were available in n = 5 trials, and ranged from 0 to 17.9 % for the ILC and from 8.5 to 34 % for the IDC.
The rate of BCTs was reported in n = 13 publications. It ranged from 17 to 72.5 % for the ILC and from 33 to 82.5 % for the IDC. The pooled BCT rate was 54.8 % (95 % CI 45.5–63.9 %) for the IDC and 35.4 % (95 % CI 26.5–45.5 %) for the ILC, according to the random effects model. The pooled OR was 2.1 (95 % CI 1.8–2.45 %, P < 0.00001; Fig. 3) according to the random effects model with no heterogeneity (I 2 = 4, P for heterogeneity 0.4).
Multivariate analysis
Only n = 3 trials reported a significant association of the ILC with the pCR, BCT or outcome. In Mathieu et al., the ILC histology was an independent poor predictor of a lower BCT but not pCR, when compared to the IDC (relative risk = 0.24, P = 0.03). Fitzal et al. reported that the IDC was associated with an increased risk of obtaining a pCR, but not a BCT or local recurrence compared to the ILC (OR = 100, P = 0.0269). In Delpech et al., the ILC was associated with an increased risk of mastectomy (OR = 1.86 compared to IDC, P = 0.01), but not a reduced risk of pCR.
Publication bias
A funnel plot and both the Begg’s and Egger’s tests were performed to assess the publication bias of the selected studies for pCR analysis. The shapes of the funnel plots showed some evidence of asymmetry (Fig. 4). However, the Begg’s test (P = 0.26 for pCR) and Egger’s test (P = 0.25) were not significant. The results remained unchanged after the leave-one-out procedure. Using the trim and fill method to account for asymmetric studies in the funnel plot had no effect on the HR for the OS. The fail-safe N was 295, indicating that it was necessary to locate and include 295 ‘null’ studies for the combined 2-tailed P value to exceed 0.050.
Discussion
This review and meta-analysis show that ductal histology is associated with a 3-fold increased chance of pCR and twofold increased possibility of breast conservation, compared to the lobular BC. These data were obtained from 17 publications that reported details of clinical and pathological responses and breast surgery after neoadjuvant chemotherapy for (large) localized or locally advanced BCs. This information is not new, because historically lobular BC has been associated with a lower response to chemotherapy and increased mastectomy rates. The population of the studies included from 50 to 100 % ER+ tumours and rare cases of HER2+ BCs (with the exception of the Untch trial which enroled 13 HER2+ ILCs). Luminal A histology in fact is a poor predictive factors for response to neoadjuvant chemotherapy. This meta-analysis was derived entirely from large retrospective databases, or from the subgroup analysis of prospective trials. The results are explained even by low-intermediate histopathological grade of ILC, with rare cases (<10 %) of high grading disease in included series. High levels of Ki 67 expression and the absence of PgR expression are also a predictive factor for obtaining a pCR [27–30]; however, unfortunately, a systematic report of pCR according to Ki 67 and PgR expression is not possible due to lack of data in almost all included studies. Lips, in his series, confirmed in fact that ILCs which were ER−/PgR− and/or HER2+ had a pCR rate of 25 % [11].
Neoadjuvant chemotherapy, in particular with the addition of taxane, increases ORR, pCR and (potentially) breast conservation when given sequentially or concomitantly with anthracycline, according to a meta-analysis published in 2008 by Cuppone et al. [31]. Our meta-analysis included patients with large localized or locally advanced BC (almost all) treated with various modern anthracycline–taxane combinations, and so the pCR estimation cannot be seriously underestimated. In BC, the ER status more than the histological subtype is widely considered to be a predictive factor for the response to chemotherapy (and for pCR), and molecular features seem to be responsible for the different responses to chemotherapy of ILC and IDC. Lips et al. analysed the protein and gene expression of BCs enroled in 2 prospective trials, and showed that the known differences in tumour characteristics between the two histological types (including ER status, HER2 status, histological grade and p53 expression) accounted for this difference, with the lowest pCR rates among the ER+/HER2− tumours in both the ILC and IDC (7 and 5 %, respectively). ILCs which were ER− and/or HER2+ had a pCR rate of 25 %. Unfortunately, the rate of pCR in the ILC subgroup, in phase III trials adopting taxane, is underreported, and always minimal. In a phase III trial comparing doxorubicin and paclitaxel with doxorubicin and cyclophosphamide, the rate of pCR for lobular histology was 0 and 8.3 % in the two arms, respectively [32].
Endocrine therapy is an alternative systemic option for primary therapy, in particular for elderly patients with comorbidities, or for those who refuse chemotherapy, specifically in ER+ BCs. In a prospective series of Dixon and colleagues, 61 patients with ILC were treated with letrozole for at least 3 months [33]. 62 patients had a clinical reduction in tumour size, and the rate of BCT was 81 %. In a phase IIb–III trial investigating the optimal duration of neoadjuvant endocrine treatment, the majority of responses were observed at month 4, with more than 70 % of the patients having undergone conservative surgery [34]. In our analysis, the rate of breast conservation in the ILCs was 35 %, apparently lower than in the endocrine neoadjuvant trials.
In another letrozole neoadjuvant study (duration of treatment 16–24 weeks), about 50 % of the patients had BCT and predictors of mastectomy at the clinical stage, inoperability at presentation, clinical stage after neoadjuvant therapy and low pathological downstage, more than in the ER status and histology [33]. These data confirm that endocrine therapy is an appropriate treatment for ER + BC, with significant conservation rates, but low or absent pCRs. A systematic review of neoadjuvant endocrine studies recently published did not report the rate of pCR in patients with lobular histologies [35]. However, rates of breast conservation in this review were higher than present results, in particular, if treatment lasted more than 3 months. This, however, could depend by stage at initiation of endocrine therapy.
In the above mentioned randomized trial, Semiglazov compared neoadjuvant aromatase inhibitors (anastrozole or exemestane for 3 months) or chemotherapy (doxorubicin + paclitaxel for 4 cycles) [10]. The rates of pCR were similar (3 vs. 6 % for endocrine vs. chemotherapy), and a greater breast conservation level was associated with the endocrine therapy (33 vs. 24 %). In this trial, however, the rates of short term toxicities were different and worse for chemotherapy. The duration of aromatase inhibitors (and chemotherapy too) in this trial was clearly suboptimal (3 months) to obtain the maximum shrinkage. The duration of neoadjuvant therapy can be crucial to obtain a pCR. It is demonstrated that a longer treatment including a non-cross resistant chemotherapy lead to a better rate of pCR. A correlation with treatment duration was not possible due to high variability in chemotherapy length. In three trials were six cycles were planned (excluding Untch trial in HER2+ setting) pCRs ranged from 2 to 11 %, in line with the pooled rate of all studies. In a recent trial prolonging, the same regimen from six up to eight cycles (in case of response) or shifting to another one (in case of non response) after two cycles of chemotherapy is associated with a better DFS in particular in hormone-receptor-positive BC [36].
A further point of discussion is the radiological evaluation of response for prediction of residual cancer burden, in particular for lobular histology. The new radiological tools as PET and MRI could help in predict residual disease and pathologic response. The first is more useful with high grade histology and ER− features (so is not proper indicated for ILC [37]), the second is more accurate of mammography, but as accurate as ultrasound in a recent meta-analysis [38]. In particular, ILC did not predict a different odds of diagnostic ratio compared to IDC.
Our meta-analysis has limitations due to the nature of the included trials. First, this is a literature-based analysis, and the majority of included trials are retrospective in nature, with one randomized trial including only 45 patients. Additionally, the lobular histology was not subject to central review to confirm morphology and biology (ER and HER2 status), but was classified in local laboratories. The patients could also be offered the physician’s choice of chemotherapy, due to younger age, better performance status, patient preference and more favourable characteristics predictive of response to chemotherapy (e.g. lower T stage at presentation, high grade, ER/PgR negative status, HER2 positive status and Ki 67 level), so the pCR rates could have even overestimated.
However, this is the first meta-analysis that confirms that ILC is less responsive to neoadjuvant chemotherapy than IDC. It includes more than 14,000 BC patients, almost all of whom were treated with contemporary chemotherapy. All included papers were also published in the last decades. Finally, the pCR definition was consistent in almost all of the cases.
In conclusion, the present systematic review shows that ILC is associated with a lower chance of obtaining pCR and breast conservation after neoadjuvant chemotherapy, compared to the IDC of the breast. Obtaining a pCR is associated with a survival benefit in BC, as in other settings. In particular, obtaining a pCR is associated with 2.45-times greater odds of survival, according to a meta-analysis recently published. Lobular cancer is per se a predictive factor of lower pCR and lower chance of breast conservation when compared to the ductal counterpart [39]. The goal of treating a large inoperable or locally advanced lobular BC is to obtain a satisfying down staging, allowing operability or conservative surgery. In the Cortazar meta-analysis of 12 neoadjuvant randomized trials, obtaining a pCR was not prognostic of event free survival in ILC [9]. Additionally, among chemotherapy or hormonal agents, the agents more suitable for primary treatment alongside have yet to be confirmed.
Other than physician preference, biology more than the simple morphology, patient age and performance status, must guide the decision in the treatment of lobular BC.
References
Orvieto E, Maiorano E, Bottiglieri L et al (2008) Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer 113:1511
Winchester DJ, Chang HR, Graves TA et al (1998) A comparative analysis of lobular and ductal carcinoma of the breast: presentation, treatment, and outcomes. J Am Coll Surg 186:416
Pestalozzi BC, Zahrieh D, Mallon E et al (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol 26:3006
Li CI, Moe RE, Daling JR (2003) Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Arch Intern Med 163:2149
Cristofanilli M, Gonzalez-Angulo A, Sneige N et al (2005) Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol 23:41
Carey LA, Dees EC, Sawyer L et al (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329
Rouzier R, Perou CM, Symmans WF et al (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678
Untch M, Fasching PA, Konecny GE et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3351
Cortazar P, Zhang L, Untch M et al. Meta-analysis Results from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC). Cancer Res 2012;72(24 Suppl):Abstract nr P1-14-20
Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Melnikova OA, Paltuev RM, Kletzel A, Berstein LM (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110(2):244–254
Lips EH, Mukhtar RA, Yau C, de Ronde JJ, Livasy C, Carey LA, Loo CE, Vrancken-Peeters MJ, Sonke GS, Berry DA, Van’t Veer LJ, Esserman LJ, Wesseling J, Rodenhuis S, Shelley Hwang E, I-SPY TRIAL Investigators (2012) Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat 136(1):35–43
Cocquyt VF, Blondeel PN, Depypere HT, Praet MM, Schelfhout VR, Silva OE, Hurley J, Serreyn RF, Daems KK, Van Belle SJ (2003) Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol 29(4):361–367
Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, Valero V, Buzdar AU, Kuerer H, Buccholz TA, Hortobagyi GN (2005) Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol 23(1):41–48
Delpech Y, Coutant C, Hsu L, Barranger E, Iwamoto T, Barcenas CH, Hortobagyi GN, Rouzier R, Esteva FJ, Pusztai L (2013) Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer 108(2):285–291
Fitzal F, Mittlboeck M, Steger G, Bartsch R, Rudas M, Dubsky P, Riedl O, Jakesz R, Gnant M (2012) Neoadjuvant chemotherapy increases the rate of breast conservation in lobular-type breast cancer patients. Ann Surg Oncol 19(2):519–526
Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, Dekhne NS (2007) Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 110(8):1687–1696
Mathieu MC, Rouzier R, Llombart-Cussac A, Sideris L, Koscielny S, Travagli JP, Contesso G, Delaloge S, Spielmann M (2004) The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer 40(3):342–351
Reitsamer R, Peintinger F, Prokop E, Hitzl W (2005) Pathological complete response rates comparing 3 versus 6 cycles of epidoxorubicin and docetaxel in the neoadjuvant setting of patients with stage II and III breast cancer. Anticancer Drugs 16(8):867–870 Erratum in: Anticancer Drugs. 2006 Mar;17(3):363
Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N, Vrancken Peeters MT (2010) The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol 17(9):2411–2418
Sullivan PS, Apple SK (2009) Should histologic type be taken into account when considering neoadjuvant chemotherapy in breast carcinoma? Breast J 15(2):146–154
Tubiana-Hulin M, Stevens D, Lasry S, Guinebretière JM, Bouita L, Cohen-Solal C, Cherel P, Rouëssé J (2006) Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol 17(8):1228–1233
Wenzel C, Bartsch R, Hussian D, Pluschnig U, Altorjai G, Zielinski CC, Lang A, Haid A, Jakesz R, Gnant M, Steger GG (2007) Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel + G-CSF. Breast Cancer Res Treat 104(1):109–114
Bollet MA, Savignoni A, Pierga JY et al (2008) High rates of breast conservation for large ductal and lobular invasive carcinomas combining multimodality strategies. Br J Cancer 98(4):734–741
Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y (2012) The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast 21(3):289–295
Pirvulescu C, Loibl S, Von Minckwitz G et al. Response pattern in 844 patients with infiltrating lobular carcinoma (ILC) of the breast after neoadjuvant chemotherapy. J Clin Oncol 2012; 30: 15 suppl, abstr 541
Vincent-Salomon A, Pierga JY, Gautier C et al (2005) Neoadjuvant chemotherapy for invasive lobular carcinomas of the breast: a poorer response rate but not a worse prognosis than invasive ductal carcinoma. Breast Cancer Res Treat 94(suppl 1):S231
Luporsi E, André F, Spyratos F et al (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132(3):895–915
Colleoni M, Bagnardi V, Rotmensz N et al (2009) Increasing steroid hormone receptor expression defines breast cancer subtypes non-responsive to preoperative chemotherapy. Breast Cancer Res Treat 116:359–369
Colleoni M, Viale G, Zahrieh D et al (2008) Expression of ER, PgR, HER1, HER2, and response: a study of preoperative chemotherapy. Ann Oncol 19:465–472
Colleoni M, Viale G, Zahrieh D et al (2004) Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res 10:6622–6628
Cuppone F, Bria E, Carlini P et al (2008) Taxanes as primary chemotherapy for early breast cancer: meta-analysis of randomized trials. Cancer 113(2):238–246
Dieras V, Fumoleau P, Romieu G et al (2004) Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 22:4958–4965
Dixon JM, Renshaw L, Dixon J, Thomas J (2011) Invasive lobular carcinoma: response to neoadjuvant letrozole therapy. Breast Cancer Res Treat 130(3):871–877
Krainick-Strobel UE, Lichtenegger W, Wallwiener D et al (2008) Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 26(8):62
Charehbili A, Fontein DB, Kroep JR et al (2013) Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: A systematic review. Cancer Treat Rev. doi:10.1016/j.ctrv.2013.06.001
von Minckwitz G, Blohmer JU, Costa SD et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E (2011) Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging 38(3):426–435
Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, von Minckwitz G, Brennan ME, Ciatto S (2013) Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst 105(5):321–333
Kong X, Moran MS, Zhang N, Haffty B, Yang Q (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47(14):2084–2090
Conflict of interest
All authors disclose any potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrelli, F., Barni, S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat 142, 227–235 (2013). https://doi.org/10.1007/s10549-013-2751-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2751-3