Abstract
Increasing ability of early breast cancer (BC) diagnosis leading to more early stage detection, better survival, and low relapse marks one of the milestones achieved over the decades. Foregoing poses a challenge for clinicians regarding optimal treatment, in which over- and under-treatment should be avoided. Classical prognostic and predictive factors fall short for individualized adjuvant therapy selection in this patient group. The key to better characterization may be found in the biology underlying individual tumors. We hypothesized that markers related to cellular proliferation and apoptosis and the balance between these two processes in tumor development will be predictive for clinical outcome. Our study population (N = 822) consisted of all early stage BC patients primarily treated with surgery in our center between 1985 and 1996. Sections of available tumor tissue (87 %, 714/822) were immunohistochemically stained for expression of p53, active-caspase-3, and Ki67. In 43 % (304/714) and 18 % (126/714) of this cohort, respectively, a biochemical C2P® risk prediction and caspase-3 assay were performed. Expression data of the mentioned markers, single, or combined, were analyzed. Results showed that both the single and combined markers, whether of apoptotic or proliferative origin had associations with clinical outcome. An additive effect was seen for the hazard ratios when data on p53, active caspase-3, and Ki67 status were combined. The assembled prognostic apoptotic–proliferative subtype showed significant association for both the overall survival (p = 0.024) and relapse-free period (p = 0.001) in the multivariate analyses of grade I breast tumors. Combined markers of tumor cell apoptosis and proliferation represent tumor aggressiveness. The apoptotic–proliferative subtypes that we present in this study represent a clinical prognostic profile with solid underlying biological rationale and pose a promising method for accurate identification of grade I BC patients in need of an aggressive therapeutic approach, thus contributing to precision medicine in BC disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of population-based screening for breast cancer (BC) with the aid of mammography led to a shift toward early-stage (<2 cm) node-negative BC detection with better prognosis [1]. This development contributed to a continuous decline in BC-related deaths, despite the increasing incidence of BC in developed countries over the past decades. Nevertheless, BC still remains one of the leading causes of cancer death in women in the western world [1].
Early diagnosis poses a challenge for clinicians regarding optimal treatment. With a relatively low relapse rate in patients detected with early BC, individual estimation of the therapeutic benefit for these patients is of crucial importance, in which over- and under-treatment has to be avoided. Defining individual tumor-specific characteristics could lend a helping hand in this consideration.
Classical prognostic and predictive factors like tumor size, histology, tumor grade, lymph node, and hormone receptor status are routinely assessed for every BC patient. Nonetheless, characterizing the tumor by identification of new or additional (bio)markers may lead to a better insight into the tumor biology and thus to its clinical behavior.
It is widely accepted that the presence of certain local factors determines tumor development, such as angiogenesis and the level of tumor cell proliferation and apoptosis. The inability to undergo apoptosis is thought to contribute to tumorigenesis and tumor progression [2]. Recent work showed that identification of the proliferation marker Ki67 proved to be of fixed prognostic value, even in an independent fashion [3, 4]. Bearing in mind that healthy tissue signifies a fine proliferative-apoptotic balance, we propose that tumor growth may be more accurately determined by the outcome of the balance between tumor cell proliferation on one side and apoptosis on the other. It is for this reason that we, in this study, aimed to identify clinically relevant biomarkers quantifying apoptosis and proliferation in breast tumors, which could be of major prognostic and predictive value. To achieve this we assessed the presence of p53, active caspase-3, and the proliferative markers Ki67 and C2P® (Sysmex Corporation, Kobe, Japan) in post-operative tumor material of early stage BC patients. Lastly, we constructed an apoptotic–proliferative subtype risk model based on the combination and rate of expressed markers. Reporting was done according to the REMARK criteria [5].
Patients and methods
Patients and tumors
Our retrospectively analyzed patient population comprised all nonmetastasized BC patients primarily treated with surgery, with or without adjuvant systemic therapy in the Leiden University Medical Center between 1985 and 1996 (N = 822). Exclusion criteria were bilateral tumors or a prior history of cancer (other than basal cell carcinoma or cervical carcinoma in situ). The following data were known: age at diagnosis, tumor grade, histologic tumor type, TNM stage, time of locoregional/distant tumor recurrence, survival time and expression of estrogen receptor (ER), progesterone receptor (PGR), and human epidermal growth factor receptor 2, HER2 [6]. Perioperative formalin-fixed paraffin-embedded tumor material was used for immunohistochemistry (IHC) and fresh frozen tumor material for biochemical assays. An experienced BC pathologist (VS) graded all tumors according to current pathological standards. All samples were handled in a coded fashion, according to the national ethical guidelines (“Code for Proper Secondary Use of Human Tissue,” Dutch Federation of Medical Scientific Societies).
Immunohistochemistry
Stainings were performed according to previously described standard protocols [7]. For each staining, all sections were stained simultaneously to avoid interassay variation. Mouse monoclonal antibodies against p53 protein (M700101 Clone D-07: Dako, NL, 0.01 M EDTA buffer [pH 8.0]) and Ki67 (M7240 Clone MIB-1: Dako, NL, 0.01 M EDTA buffer [pH 8.0]) were used. For active caspase-3 detection, an immunohistochemical staining was performed with antibodies directed against cleaved caspase-3 (anti-Asp175 #9661: Cell Signaling, USA, citrate buffer 0.1 M [pH 6.0]). Tonsil and colorectal carcinoma sections served as positive control for p53, Ki67 and active caspase-3 staining, respectively. Negative controls underwent the whole immunohistochemical staining without primary antibodies.
Evaluation of immunostaining
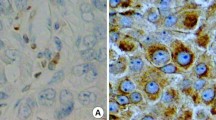
Two independent observers performed quantification of p53-, active caspase-3-, and Ki67-positive stained cells in a blinded manner. For p53, the percentage positive stained nuclei of tumor cells were microscopically assessed by determining the mean percentage in all three punches of the TMA. Categorization was made by dividing the mean percentage scores into: wild type (≤50 % positive nuclei in the tumor material) and mutant pattern of staining (>50 % expression of tumor nuclei stained positive for p53; Fig. 1a) [8].
a Immunohistochemical p53 staining, left wild type staining pattern (≤50 % of nuclei), right mutant staining pattern (>50 %) of the tumor nuclei. b Immunohistochemical active caspase-3 staining, from left to right negative (<0.49 positive cells), low (0.5–1.49 positive cells), intermediate (1.5–2.49 positive cells), and high (>2.49 positive cells) expression staining in human breast tumor (cut-off points: mean expression of active caspase-3 in three breast cancer tissue cores). c Immunohistochemical Ki67 staining, left absent (0 %), and right present (>1 %) expression staining in human breast tumor
For active caspase-3, the mean expression grade of positively stained cells in the TMA was defined: absent (expression grade: 0–0.49 positive cells), low (expression grade: 0.5–1.49 positive cells), intermediate (expression grade: 1.5–2.49 positive cells), and high scores, corresponding with a mean expression of >2.5 positive cells in the tumor material (Fig. 1b).
Ki67 expression was divided into absent (0 %) and present (>1 %) positively stained nuclei, based on the mean percentage of all three-tumor punches per patient (Fig. 1c).
C2P® risk prediction score assay
C2P® risk prediction scores (C2P®-RS) are a proliferation assay developed by Sysmex Corporation which is based on cyclin-dependent kinase (CDK)-1 and -2, both playing a pivotal role in cell cycle regulation [9]. RSs are based not only on CDK-1 and -2 presences in the tumor material, but also on the enzyme activity rate [9]. CDK-1 and -2 assays were performed using frozen tissue samples. Subsequently, the C2P®-RS was calculated using a predetermined formula, after which the tumors were divided into three categories (high, intermediate, and low RS groups; [9]). For a detailed assay protocol see manuscript by Kim et al. [9].
Active caspase-3 assay
Biochemical quantification of active caspase-3 was determined in 18 % of the BC patients (126/714). The enzymatic activity of caspase-3 was obtained by lysing ten 10-μm thick cryostat sections per sample in 500 μL lysis buffer containing 10 mM HEPES, pH 7.0, 40 mM β-glycerophosphate, 50 mM NaCl, 2 mM MgCl2, and 5 mM EGTA, followed by 10 min of homogenization using a Polytron homogenisator (PT-MR 2100, Kinematica, Luzern, Switzerland) and four freeze–thaw cycles before storing it at −80 °C. Protein concentration was determined using the Bradford method [10]. For measurements of caspase-3 enzyme activity, 50 μL of each sample was incubated with 5 μL of 1 mM substrate Ac-DEVD-AFC (A0466-1MG, Sigma Aldrich, USA) in a 100 mM HEPES buffer, pH 7.25, containing 10 % sucrose, 0.1 % (v/v) Nonidet-P40, and 10 mM dithiothreitol (D0632, Sigma Aldrich, USA) for 2 h at 37 °C. During incubation at 37 °C, fluorescent AFC was cleaved off by active caspase-3, corresponding with the level of caspase-3-activity per sample. Fluorescent AFC absorbance was monitored in a fluorometer equipped with a 400 nm excitation filter and 505 nm emission filter at time-point: 00.00 h and again at time-point: 02.00 h. Calibration curves were prepared by plotting the values of free-AFC standard absorbance versus concentration in nmol/L. Caspase-3 activity was indicated in pmol AFC/min/mg protein.
Statistical analysis
Statistical analyses were performed using the statistical package SPSS (version 20.0 IBM SPSS Statistics). Patients with missing data, mostly due to material handling were excluded from statistical analysis. Cohen’s κ coefficient was used to assess the interobserver agreement in quantification of p53, active caspase-3, and Ki67 expression. The χ 2 test was used to evaluate associations between various clinicopathological parameters and apoptotic and proliferative markers in the tumor material. The clinical endpoints examined were relapse-free period (RFP) defined as the time from surgery until an event (locoregional recurrence and/or a distant recurrence, whichever came first), and overall survival (OS) defined as the time from surgery until death by any reason. The Kaplan–Meier method was used for survival plotting and log-rank test for comparison of RFP and OS curves. Cox proportional hazard analysis was used for univariate and multivariable analysis for RFP and OS. Variables with a p value of <0.1 in univariate analysis were entered in multivariable analysis.
In order to compare the agreement of the different techniques used for caspase-3 (IHC and biochemical assay) estimation, a Spearman’s ρ correlation test was performed.
Results
Patient and tumor characteristics
Perioperative tumor material was available of 87 % (714/822) of the patients. The median age of this cohort was 58 years (range 23–96 years) with a median follow-up of 10 years (range 0.02–22 years; clinicopathological characteristics: Tables 1, 2). Good interobserver agreement was seen (≥0.6) using the Cohen’s κ coefficient for quantification of immune-stained markers.
p53 expression
Immunohistochemical data for p53 expression were available for 80 % (574/714) of the patients. Mutant p53 was significantly present in patients with more advanced pathological tumor stages (p < 0.001), more advanced TNM stage (p = 0.033), higher tumor grades (p < 0.001), and ductal tumors (p = 0.017; Table 1). Tumors with adverse hormonal characteristics: ER negative (−), PGR negative (−), and HER2 positive (+) are significantly associated with mutant p53 protein (ER: p = 0.013, PGR: p = 0.004, and HER2: p < 0.001; Table 1).
Analysis of the OS showed a statistical significant association between mutant p53 and survival outcome of patients (p < 0.001, hazard ratio (HR) 2.150, 95 % confidence interval (CI) 1.549–2.983; Table 3), also remaining an independent prognostic marker in multivariable analysis (p = 0.009, HR 1.776, 95 % CI 1.158–2.726). The explanation hereof lies in the fact that mutated p53 protein cannot be cleared away in the tumor cell leading to high amounts of inactive p53 stacking which is often seen to a greater extent in more aggressive tumor types, since no apoptosis is induced [11]. For RFP, a significant relation was seen for mutant p53 in the univariate analysis only (p = 0.002, HR 1.838, 95 % CI 1.255–2.692; Fig. 2a; Table 4).
Active caspase-3 expression
Data of active caspase-3 IHC were available for 80 % (575/714) of the BC patients. Tumors in which determination of both the active caspase-3 IHC expression and caspase-3 biochemical enzymatic activity was performed (N = 106), comparison analyses showed excellent agreement (p = 0.011). There was significant association between active caspase-3 expression in IHC and higher pathological tumor stage (p < 0.001), more advanced TNM stage (p < 0.001), higher tumor grade (p < 0.001), ductal tumor histology (p < 0.001), and a statistical trend was seen for lymph node involvement (p = 0.065; Table 1). ER−, PGR−, and HER2 over-expressing tumors are related to high caspase-3 expression with p values of <0.001, 0.002 and 0.002, respectively (Table 1). Additional analyses showed a close relationship between caspase-3 expression and Ki67 expression in the same tumor material (p = 0.001, data not shown), indicating that proliferation and apoptosis are closely linked within the tumor and thus should be accounted for if one seeks optimal prognostic-predictive value determination. Survival analysis showed that a higher caspase-3 expression is significantly associated with worse OS (p < 0.001, HR 1.908, 95 % CI 1.407–2.588; Table 3), however, not remaining an independent prognostic factor after multivariate correction (p = 0.414). For RFP, a significant relation was found for high caspase-3 expression and relapse rate (p < 0.001, HR 1.943, 95 % CI 1.356–2.783; Fig. 2b), again not maintaining individual prognostic value in the multivariate correction (p = 0.366; Table 4).
Ki67 expression
Ki67 expression data were available for 78 % (556/714) of the patients. No relation was seen for Ki67 expression in the tumor and tumor stage or nodal involvement (Table 2). However, for high tumor grades and tumors of ductal histology (both p < 0.001), PGR− (p = 0.01) and HER2 over-expressing tumors (p = 0.019), a significant association was found with high Ki67 expression, corresponding with a high proliferative rate (Table 2). A statistical trend was seen for TNM stage and high Ki67 expression (p = 0.066).
Patients with high Ki67 tumor expression had worse OS (p = 0.007, HR 1.348, 95 % CI 1.086–1.673), however, losing its significance in the multivariate correction (p = 0.564; Table 3). A significantly higher relapse rate was noted for high Ki67 expression compared to low proliferation rate in the tumor material (p = 0.021, HR 1.339, 95 % CI 1.045–1.716; Fig. 2c). High Ki67 did not remain significantly associated with a higher relapse rate in the multivariate correction (p = 0.269; Table 4).
C2P® risk prediction score
Data previously published by our group already described the C2P®-RS as a promising prognostic marker in early BC patients [12]. Using the same cohort, 43 % (304/714) of the patients had tumor material available for C2P® analyses. Significance was found for high C2P® risk score and tumor grade III scores (p = 0.004), young age (<55 years of age, p = 0.020) and ER+ tumors (p = 0.049; Table 2). A statistical trend was seen for TNM stage (p = 0.052).
No statistical relation was seen for C2P® and OS (p = 0.263; Table 3). High C2P® risk scores were significantly associated with higher relapse rates (p = 0.026, HR 1.953, 95 % CI 1.199–3.181), however, not maintaining its significance in the multivariate correction (Fig. 2d; Table 4).
Results: combined IHC data
p53–Ki67
From 72 % (516/714) of the patients, immunohistochemical data were available for both the p53 and Ki67, making them eligible for the determination of the prognostic value of a combined p53–Ki67 marker. Significance was found in relation with OS and RFP, where high Ki67 combined with mutant p53 expression had the worse clinical outcome (OS: p < 0.001, HR 2.458, 95 % CI 1.654–3.655 [Table 5] and RFP: p = 0.003, HR 2.307, 95 % CI 1.479–3.598 [Table 6]) compared to a HR of 1.00 in low Ki67 combined with wild type p53 protein expression. All other combinations of p53 and Ki67 data showed HRs ranging between >1.00 and <2.396 for the OS and >1.00 and <1.327 for the RFP. However, in the multivariate analysis for OS, only the combination of low Ki67 expression and mutant p53 remained significant (OS: p = 0.037, Table 5).
When we compared the highest HRs of the single markers for p53 (OS HR 2.150 and RFP HR 1.838) and Ki67 (OS HR 1.348 and RFP HR 1.339), we concluded that by combining these two markers in one combination (p53–Ki67), we induce additive strength to his prognostic-predictive marker, leading to a higher HR (OS HR 2.458 and RFP HR 2.307) than the single biomarker HRs (Tables 7, 8).
p53–active caspase-3
Seventy four percent (529/714) of the patients had both the p53 and active caspase-3 IHC data available. Again for both the OS and RFP, significance was found with the combined p53–caspase-3 biomarker. Mutant p53 protein expression combined with high active caspase-3 expression resulted in the highest HR for death in OS (p < 0.001, HR 3.012, 95 % CI 2.044–4.439; Table 5) and the RFP (p < 0.001, HR 2.673, 95 % CI 1.703–4.195; Table 6). For the OS, this remained an independent prognostic biomarker after multivariate correction (p = 0.037, HR 2.008, 95 % CI 1.241–3.249; Table 5).
Again a higher HR (OS HR 3.012 and RFP HR 2.673) was seen when patients with the clinically most adverse expression pattern of single markers p53 (OS HR 2.150 and RFP HR 1.838) and active caspase-3 (OS HR 1.908 and RFP HR 1.943) were compared to the HR of the combined p53–caspase-3 marker, indicating the probability of an additive quality (Tables 7, 8).
Ki67–active caspase-3
Data of both the Ki67 and active caspase-3 expression were available from 33 % (239/714) of the patients of this cohort. Both the high expression of Ki67 and active caspase-3 had a significant worse OS (p < 0.001, HR 3.012, 95 % CI 2.044–4.439; Table 5) and RFP (p < 0.001, HR 2.258, 95 % CI 1.599–3.189; Table 6) compared to low Ki67 with low caspase-3 expression (Fig. 3a, b). In the multivariate analyses, neither the RFP (p = 0.156) nor the OS (p = 0.676) remained an individual prognostic marker. Again additive properties were seen for the combined biomarker: Ki67–active-caspase-3 (OS HR 2.137 and RFP HR 2.258), compared to the single biomarkers (Ki67: OS HR 1.348 and RFP HR 1.339; caspase-3: OS HR 1.908 and RFP HR 1.943; Tables 7, 8).
a Relapse free period (RFP) curves for combined analysis of active caspase-3 and the proliferative marker Ki67. Both single markers were grouped into low or high expression in the tumor tissue (for active caspase-3 the division was made based on the RFP curve seen in Fig. 2b) after which they were combined. b The same was done for the overall survival (OS) curves for this combined marker
C2P® in combination with p53 or active caspase-3 or Ki67
Neither p53 (20 % [142/714]), active caspase-3 (21 % [147/714]) nor Ki67 (21 % [150/714]) combined with C2P®-RS showed a statistical significant relation with outcome.
Apoptotic–proliferative tumor subtype
Due to the supporting outcome of the combined markers, we constructed a prognostic model based on the expression pattern of the three risk contributing markers: p53, active caspase-3, and Ki67 (488/714, 68 %). C2P® was not included in this model due to the limited number of patients in whom this marker was determined (frozen tumor tissue was needed), leading to lack of power in the combined analysis. Expression scores of these markers were dichotomized. For all patients, one point was allocated for each marker expressed, indicating one risk factor present; resulting in a score of 0 for patients without expression of any marker and a score of 3 for patients with all markers highly expressed. The apoptotic–proliferative subtype model was significantly associated with the molecular subtype of the tumor, in which higher apoptotic–proliferative scores were related to more aggressive molecular tumor subtypes (HER2+ type and basal like) and negative to low apoptotic–proliferative scores to the less aggressive Luminals A and B molecular tumor subtypes (p < 0.001).
For the OS (p < 0.001, score 1: HR 1.569 [95 % CI 1.171–2.103], score 2: HR 1.922 [95 % CI 1.386–2.667], score 3: HR 3.657 [95 % CI 2.297–5.822]) and RFP (p < 0.001, score 1: HR 1.468 [95 % CI 1.046–2.061], score 2: HR 2.122 [95 % CI 1.473–3.059], score 3: HR 3.058 [95 % CI 1.792–5.218]), significant univariate association was found (Fig. 4). When the cohort was split on tumor grade, we found a significant association in the multivariate corrected analyses for both the OS (p = 0.024) and RFP (p = 0.001) for only grade I tumors (Fig. 4; Tables 5, 6). When the cohort was split on TNM stage, we found that only stages I and IIA patients had a significant outcome in the univariate OS analysis for the apoptotic–proliferative subtype model. This remained borderline significant in the multivariate corrected analysis for OS in TNM stage I patients (p = 0.056; Table 5).
Results: biochemical assay active caspase-3
Eighteen percent (126/714) of the patients had frozen material available for a biochemical caspase-3 assay. For analysis, outcomes were converted into a categorical parameter (< and >the median value [2.74 pmol AFC/min/mg protein]). In the univariate analyses, neither for OS (p = 0.7) nor RFP (p = 0.5), a significant relation was found herewith.
When caspase-3 assay data were combined with the C2P® data (75/714, 10.5 %), a significant association was found for the C2P® risk prediction and the dichotomized biochemical caspase-3 expression (low/high). Results showed that high C2P® was significantly associated with high biochemical caspase-3 expression. However, there was no significant relation regarding OS (p = 0.670) or RFP (p = 0.628) for this combination (data not shown).
Next, we calculated the ratio among CDK-1 activity, a crucial contributor of the C2P® biomarker, and biochemical caspase-3 (107/714, 15 %). The ratio was transformed in a dichotomous variable by use of the median value due to a skewed distribution. Significant associations, in the favor of weaker proliferative characteristics of the tumor, were seen in the RFP (p = 0.016) and OS (p = 0.014), both maintaining their significance in the multivariable analyses (RFP: p = 0.009, HR 2.460, 95 % CI 1.248–4.849 and OS: p = 0.015, HR 2.137, 95 % CI 1.161–3.934 (Tables 5, 6, respectively)). Combined CDK-2 and biochemical caspase-3 (115/174, 16.1 %) only showed a significant association in the RFP (p = 0.003) in favor of a higher apoptotic rate, remaining an independent factor after multivariate correction with p value = 0.012, HR 2.501, 95 % CI 1.228–5.096 (Table 6).
Discussion
Over the last few years, the impact of single apoptotic and proliferative markers on tumor progression and patient outcome in BC was thoroughly investigated, but often showed contradictory results [13–15]. An explanation could be the misinterpretation that emanates from single apoptotic and proliferative marker expression due to the fact that they do not reflect the interaction with one another. In this manuscript, we circumvented this shortcoming by combining dual markers and constructed an apoptotic–proliferative subtype model, in which all important markers were incorporated to prevent misinterpretation of these closely linked pathways.
It is hypothesized that the imbalanced presence of apoptosis and proliferation is a hallmark for tumor aggressiveness. Consequently, this apoptotic–proliferative misbalance results in either progression or inhibition of tumor growth depending on the direction of the outcome of the balance.
For both the single and combined markers, independent of being a proliferative or apoptotic marker, high expression rates are associated with higher HRs, in which the majority of combined markers have an additive effect on one another leading to higher HRs.
For active caspase-3, our data showed counter intuitive worse clinical outcome when highly expressed, thus corresponding with a high apoptotic rate in the tumor [2]. Combined analyses demonstrated that this poor outcome was associated with high proliferative Ki67 presence in the breast tumor, being a good example of how single marker experiments can be misinterpreted. It should be clear that the high proliferative Ki67 marker apparently dominates the clinical outcome of these high active caspase-3 expressing tumors. It could be considered that the apoptotic marker can merely keep up with the high proliferation rate of the tumor, resulting in excess proliferation, consequently leading to progression of the BC. Nevertheless, this difference in apoptosis induction in tumors expressing high levels of Ki67 is also a tumor characteristic worthy of observation and serves as an excellent marker for more accurate prognostication. The combined high apoptosis–high proliferation relation seen in this study was also seen in work done by Parton et al. [16].
Biochemical assay data retrieved from this study strengthens the conclusion found in IHC focusing on combined marker analyses. Our assay results are supported by data from Zeestraten et al. [17] whom also showed the high prognostic value of CDK-1 in stage II colon cancer patients.
By constructing an apoptotic–proliferative tumor subtyping model, we demonstrated that the combination of the expression rates of all relevant apoptotic and proliferative markers leads to a valuable prognostic indicator in grade I breast tumors. To the best of our knowledge, we are the first group providing such detailed insight in the tumor apoptosis and proliferation ratio in BC, showing that this cell proliferative and death ratio are of crucial value compared to single marker interpretation in the control of tumor progression and therefore, in determining patient prognosis. Results of this study lead to assume that apoptotic–proliferative subtyping in grade I tumors could be of crucial importance in identifying patients with a low tumor grade with an increased risk of poor prognosis, being those containing the most detrimental apoptotic–proliferative marker combination. With the increased tendency of earlier diagnosis due to better BC awareness and the introduction of population based screening, it comes as no surprise that the BC incidence has tilted to more early stage, low grade breast tumors [18]. Introducing our newly designed apoptotic–proliferative tumor subtyping model will lead to targeted selection of the grade I BC patients that would truly benefit of an aggressive therapeutic regime due to an adverse apoptotic–proliferative balance. In the current state of affairs, where over- and under-treatment leads to considerable debate in clinical practice, identification of patient groups for implementation of personalized therapy will become increasingly important.
This cohort consisted of BC patients diagnosed and treated between 1985 and 1996, this time frame also marking the beginning of adjuvant hormonal therapy which led to less protocolled regimes and documentation hereof. Also, the chemotherapy given at that time point clearly does not meet today’s standards, and therefore, no clinical consequence could be deduced. Despite these shortcomings, this study clearly states high prognostic value. Further research should validate our findings and focus on the predictive value in light of today’s therapeutic standards.
References
Weigel MT, Dowsett M (2010) Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 17:R245–R262
O’Donovan N, Crown J, Stunell H et al (2003) Caspase 3 in breast cancer. Clin Cancer Res 9:738–742
Pathmanathan N, Balleine RL (2013) Ki67 and proliferation in breast cancer. J Clin Pathol 66(6):512–516
Gong P, Wang Y, Liu G, Zhang J, Wang Z (2013) New insight into Ki67 expression at the invasive front in breast cancer. PLoS ONE 8:e54912
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
van Nes JG, de Kruijf EM, Faratian D et al (2011) COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Breast Cancer Res Treat 125:671–685
de Kruijf EM, van Nes JG, Sajet A et al (2010) The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res 16:1272–1280
Nout RA, Bosse T, Creutzberg CL et al (2012) Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/beta-catenin and P53 pathway activation. Gynecol Oncol 126:466–473
Kim SJ, Nakayama S, Shimazu K et al (2012) Recurrence risk score based on the specific activity of CDK1 and CDK2 predicts response to neoadjuvant paclitaxel followed by 5-fluorouracil, epirubicin and cyclophosphamide in breast cancers. Ann Oncol 23:891–897
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Nyiraneza C, Jouret-Mourin A, Kartheuser A et al (2011) Distinctive patterns of p53 protein expression and microsatellite instability in human colorectal cancer. Hum Pathol 42:1897–1910
van Nes JG, Smit VT, Putter H et al (2009) Validation study of the prognostic value of cyclin-dependent kinase (CDK)-based risk in Caucasian breast cancer patients. Br J Cancer 100:494–500
Jager JJ, Jansen RL, Arends JW (2002) Clinical relevance of apoptotic markers in breast cancer not yet clear. Apoptosis 7:361–365
Oh YL, Choi JS, Song SY et al (2001) Expression of p21Waf1, p27Kip1 and cyclin D1 proteins in breast ductal carcinoma in situ: relation with clinicopathologic characteristics and with p53 expression and estrogen receptor status. Pathol Int 51:94–99
Ross JS, Linette GP, Stec J et al (2003) Breast cancer biomarkers and molecular medicine. Expert Rev Mol Diagn 3:573–585
Parton M, Krajewski S, Smith I et al (2002) Coordinate expression of apoptosis-associated proteins in human breast cancer before and during chemotherapy. Clin Cancer Res 8:2100–2108
Zeestraten EC, Maak M, Shibayama M et al (2012) Specific activity of cyclin-dependent kinase I is a new potential predictor of tumour recurrence in stage II colon cancer. Br J Cancer 106:133–140
Esserman L, Shieh Y, Thompson I (2009) Rethinking screening for breast cancer and prostate cancer. JAMA 302:1685–1692
Acknowledgments
Research support: Dutch Cancer Society (KWF 2007-3968).
Disclaimer
None of the authors who contributed to this article has any financial or personal relationships with people or organizations that could inappropriately influence the data published.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hideki Ishihara no longer affiliated with Sysmex Corporation, Kobe, Japan.
Rights and permissions
About this article
Cite this article
Engels, C.C., Ruberta, F., de Kruijf, E.M. et al. The prognostic value of apoptotic and proliferative markers in breast cancer. Breast Cancer Res Treat 142, 323–339 (2013). https://doi.org/10.1007/s10549-013-2748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2748-y