Abstract
The aim of this study was to analyze the correlation between the pathologic complete response (pCR) rate after neoadjuvant chemotherapy and long-term outcome (distant metastases-free survival [DMFS]) in patients with early-stage breast cancer using BluePrint and MammaPrint molecular subtyping versus clinical subtyping using immunohistochemistry/fluorescence in situ hybridization (IHC/FISH) for the determination of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 (HER2). Data were analyzed from 437 patients in four neoadjuvant chemotherapy trials. BluePrint and MammaPrint outcomes were determined from 44K Agilent arrays, the I-SPY 1 data portal, or Affymetrix U133A arrays. The pCR rate differed substantially among BluePrint molecular subgroups: 6 % in Luminal A-type, 10 % in Luminal B-type, 47 % in HER2-type, and 37 % in Basal-type patients. In the Luminal A-type group (n = 90; including seven HER2-positive patients and eight triple-negative patients by IHC/FISH), the 5-year DMFS rate was 93 %. The pCR rate provided no prognostic information, suggesting these patients may not benefit from chemotherapy. Forty-three of 107 (40 %) HER2-positive patients were classified as Luminal-type by BluePrint and may have lower response rates to targeted therapy. Molecular subtyping identified 90 of 435 (21 %) patients as Luminal A-type (BluePrint Luminal-type/MammaPrint Low Risk) with excellent survival. The pCR rate provided no prognostic information. Molecular subtyping can improve the stratification of patients in the neoadjuvant setting: Luminal A-type (MammaPrint Low Risk) patients have a good prognosis with excellent survival and do not seem to benefit from chemotherapy. We observed marked benefit in response and DMFS to neoadjuvant treatment in patients subtyped as HER2-type and Basal-type. BluePrint with MammaPrint molecular subtyping helps to improve prognostic estimation and the choice of therapy versus IHC/FISH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy is increasingly being used for the treatment of early-stage breast cancer (ESBC) and particularly locally advanced (LABC) to enable breast preservation and improve surgical outcomes [1–4] compared to postoperative chemotherapy, with equivalent recurrence rates, survival, and locoregional control [3]. The classification of breast cancers into molecular subtypes is important for the appropriate selection of neoadjuvant therapy in patients with ESBC [5]. Previous studies have shown that patients with different breast cancer subtypes have distinct clinical outcomes [6–9].
The pathologic complete response (pCR) rate has been used as a primary endpoint in numerous neoadjuvant clinical trials [10, 11] and a number of large randomized trials have shown that the pCR rate can serve as a surrogate predictor for long-term outcome [12–14]. This observation is consistent across trials, even when patient populations and the definition of the pCR differ. The US Food and Drug Administration (FDA) recently issued draft guidance for industry on the use of the pCR as a clinical-trial endpoint for accelerated drug approval for neoadjuvant treatment of high-risk ESBC [10, 15]. In addition, breast cancer patient advocates have recognized the importance of a pCR in guiding treatment decisions in neoadjuvant clinical trials [16]. The beneficial effects of neoadjuvant chemotherapy are greatest in patients with human epidermal growth factor receptor-2 (HER2)-positive and triple-negative tumors [11, 17, 18]. Subtyping may become even more important in HER2+ cancers with the availability of novel dual-targeting strategies [19–21].
The classification of breast cancers into molecular subtypes was originally developed using gene-expression array analysis [5, 22], e.g., BluePrint [23] and PAM50 [24, 25]. Initially, simple methods for the subclassification of breast cancer evolved using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) [26, 27]. While a variety of gene-expression profiling methods have been pursued, currently there is no agreement on which molecular profile is best for discriminating between breast cancer subtypes to show differences in clinical outcomes, including time-dependent endpoints. BluePrint was developed to provide an additional method for the molecular subclassification of breast cancer. The profile was developed using a rational-based method to insure a robust and reproducible profile with concordant IHC/FISH-assessed samples for the estrogen receptor (ER), progesterone receptor (PR), and HER2. BluePrint determines the mRNA levels of 80 genes that best discriminate between Luminal-type, HER2-type, and Basal-type tumors and was validated using four independent validation cohorts consisting of 784 patients [24]. The Luminal subtype can be further divided into type A (low risk) and type B (high risk) [28] using a validated profile, e.g., the 21-gene recurrence score OncotypeDX [29] or the 70-gene profile MammaPrint [30].
Both OncotypeDX and MammaPrint have been studied in the neoadjuvant setting [29, 30]. OncotypeDX was positively associated with the likelihood of a pCR (P = 0.005) in a study of 89 patients with LABC [29]. In a study of 167 patients with LABC, no Low-Risk MammaPrint patients achieved a pCR (0 of 23), whereas 29 of 144 (20 %) High-Risk patients did (P = 0.015) [30]. Thus, a pCR is unlikely to be achieved in patients with tumors that have a Low-Risk MammaPrint, whereas patients with High-Risk tumors are sensitive to chemotherapy [30]. By combining BluePrint and MammaPrint, Luminal-type cancers can be further stratified into A-type (BluePrint Luminal-type and MammaPrint Low Risk) and B-type (BluePrint Luminal-type and MammaPrint High Risk). This distinction is important for determining prognosis and can guide the decision whether or not to use chemotherapy as neoadjuvant or adjuvant treatment.
In this study, the molecular stratification of patients with BluePrint and MammaPrint was used to correlate the response to neoadjuvant chemotherapy and long-term outcomes in patients with ESBC or LABC and the results compared with those obtained by classification using IHC/FISH for ER, PR, and HER2.
Patients and methods
Neoadjuvant studies
This retrospective analysis was performed on samples from 437 patients enrolled in four independent neoadjuvant chemotherapy clinical trials: 144 patients from the I-SPY 1 trial [31]; 131 patients [32] and 99 patients [33] from two biomarker discovery trials at the University of Texas M.D. Anderson Cancer Center; and 63 patients from the City of Hope National Medical Center [34]. In two trials, a pCR was defined as no invasive or noninvasive residual disease in the breast or axillary lymph nodes [31, 34]; in the other trials, the definition included noninvasive breast residual disease [32, 33]. A recent review showed that residual noninvasive cancer does not contribute negatively to recurrences or long-term outcomes [21].
In the I-SPY 1 trial, all patients received doxorubicin plus cyclophosphamide as initial chemotherapy and all except four subsequently received a taxane [31]. Of the 45 patients with HER2-positive tumors, 14 patients received neoadjuvant and 23 patients received adjuvant trastuzumab (the other patients were enrolled before trastuzumab approval and therefore did not receive concurrent neoadjuvant trastuzumab) [31]. In the M.D. Anderson Cancer Center studies, patients received preoperative chemotherapy with sequential paclitaxel (80 mg/m2 weekly for 12 cycles) and 5-fluorouracil, doxorubicin, and cyclophosphamide (500, 50, and 500 mg/m2, respectively, every 21 days for four cycles) [32, 33]. In the City of Hope randomized phase II study, patients received six cycles of docetaxel 75 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2 every 21 days (TAC) versus doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for four cycles, followed by carboplatin at an AUC of 2 and nab-paclitaxel 100 mg/m2 weekly for 3 weeks every 28 days (ACAC). A separate stratum of HER2-positive patients received ACAC and trastuzumab as a 4 mg/kg loading dose then 2 mg/kg weekly for 12 weeks as neoadjuvant therapy [34]. Of the patients with sufficient quality and quantity of RNA included in this analysis, 17 patients received TAC, 23 patients received ACAC, and 23 patients received ACAC with the addition of trastuzumab to carboplatin and nab-paclitaxel as a 4 mg/kg loading dose followed by trastuzumab 2 mg/kg weekly for 12 weeks. Of the 23 patients who received ACAC, 22 patients were classified as HER2-positive by IHC/FISH and one patient was initially diagnosed as HER2-positive; however, re-assessment of the initial biopsy revealed HER2-negative disease.
Molecular subtyping
BluePrint and MammaPrint outcomes were derived from either 44K Agilent arrays analyzed at Agendia according to the manufacturer’s protocols [34], were available through the I-SPY 1 data portal [35], or were determined from Affymetrix U133A arrays [32, 33]. Expression data were quantified using Feature Extraction software. Four distinct molecular subgroups—Luminal A-type, Luminal B-type, Basal-type, and HER2-type—were identified and used for further analysis. In this study, we defined Luminal A-type tumors as Luminal type by BluePrint with a Low-Risk score by MammaPrint and Luminal B-type tumors as BluePrint Luminal type with a MammaPrint High-Risk score.
Stratification using IHC/FISH for ER, PR, and HER2
In order to compare molecular subtyping with currently used diagnostic classification, outcomes were analyzed using IHC/FISH for ER, PR, and HER2 in the following three groups: triple-negative (ER-negative, PR-negative, HER2-negative), hormone receptor (HR)-positive (ER-positive and/or PR-positive, HER2-negative), and HER2-positive. HR and HER2 status were determined by IHC/FISH in the diagnostic core needle biopsy specimens before chemotherapy. In seven patients, the ER, PR, or HER2 data were unavailable. In the I-SPY 1 trial, HR status was determined by IHC and considered positive if the Allred score was >3; HER2 status was determined by IHC and/or FISH assays locally and centrally at the University of North Carolina. HER2 status was regarded as positive if there was 3+ staining and/or FISH-positive (defined as a HER2:CEP17 ratio > 2.2) locally or centrally. In the M.D. Anderson Cancer Center studies, cancers with ≥10 % positive nuclear staining for ER or PR with IHC were considered as HR-positive. Specimens that showed either 3+ IHC staining for HER2 or had a HER2 gene copy number ≥2.0 by FISH were considered HER2-positive. In the City of Hope study, patients with HR-positive disease had positive expression of HRs (IHC ≥ 1 %) and were not overexpressing HER2 by IHC (0–1) or, in the case of an IHC of 2, were negative by FISH or by alternative gene testing. Patients with HER2 3+ staining by IHC or gene amplification (FISH or alternative gene testing) were considered HER2-positive.
Outcomes
The response to neoadjuvant chemotherapy was defined by the pCR according to the definitions used in the clinical trials [31–34]. Long-term outcome was defined as the 5-year distant metastases-free survival (DMFS) rate. The response to neoadjuvant chemotherapy and long-term outcomes were also analyzed for patients treated with and without trastuzumab HER2-targeted therapy. The response to treatment was analyzed for patients classified as HER2-type by BluePrint, for HER2-positive IHC/FISH patients classified as Luminal-type, and for all HER2-positive IHC/FISH-positive patients.
Results
The clinical characteristics of the 437 patients (age range 26–79 years) are shown in Table 1. Molecular subtyping classified 90 (21 %) patients as Luminal A-type, 154 (35 %) patients as Luminal B-type, 70 (16 %) patients as HER2-type, and 123 (28 %) patients as Basal-type. Stratification using IHC/FISH for ER, PR, and HER2 identified 219 (53 %) patients as HR-positive, 107 (26 %) patients as HER2-positive, and 104 (25 %) patients as triple-negative.
Overall, the pCR rate was 23 % (99 of 437 patients) but differed substantially in the different molecular subgroups: Luminal A-type, 6 %; Luminal B-type, 10 %; HER2-type, 47 %; and Basal-type, 37 %. Table 2 shows the data for the pCR versus residual disease, prognosis (5-year DMFS rate), and benefit from chemotherapy (5-year DMFS rate in patients with and without a pCR) in the different patient subgroups classified by molecular subtyping compared with IHC/FISH for ER, PR, and HER2. The 5-year DMFS rate was 69 % in the triple-negative group and 81 % in the HR-positive group (Fig. 1a; Table 2). In patients classified by molecular subtyping, the 5-year DMFS was 68 % in the Basal-type subgroup and 93 % in the Luminal A-type subgroup (Fig. 1b; Table 2). More patients were classified as Basal-type (n = 123) versus IHC/FISH determination of triple-negative (n = 104) but the pCR rate was similar (Basal-type 37 %, triple-negative 34 %) (Table 2).
Survival rates according to stratification based on a IHC/FISH for ER, PR, and HER2 and b molecular subtyping using BluePrint and MammaPrint. IHC immunohistochemistry, FISH fluorescence in situ hybridization, ER estrogen receptor, PR progesterone receptor HER2 human epidermal growth factor receptor-2
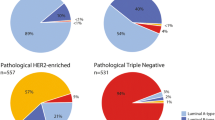
Patients with Luminal A-type cancers had a good prognosis, with excellent survival despite a very low pCR rate (6 %) and no apparent benefit from chemotherapy. Fig. 2 shows the prognosis for patients with and without a pCR in different subgroups classified according IHC/FISH for ER, PR, HER2 (Fig. 2a–c, respectively), and molecular subtyping (Fig. 2d–g, respectively). In patients with triple-negative breast cancer, the pCR was indicative of a good long-term outcome with a 5-year DMFS rate 88 versus 59 % in patients with residual disease. In patients classified by molecular subtyping, the pCR versus residual disease resulted in a similar prognosis for good long-term outcome in the Basal-type group (91 versus 54 %). Patients classified with HER2-type breast cancer and a pCR had a 5-year DMFS rate of 91 versus 88 % for patients classified as HER2-positive by IHC/FISH with a pCR (Fig. 2b, f; Table 2).
Prognosis after pCR by IHC/FISH assessment and BluePrint/MammaPrint molecular subtyping a HR-positive, b HER2-positive, c Triple-negative, d BluePrint/MammaPrint Luminal A-type, e BluePrint/MammaPrint Luminal B-type, f BluePrint HER2-type, g BluePrint Basal-type. pCr pathologic complete response, IHC immunohistochemistry, FISH fluorescence in situ hybridization, HR hormone receptor, HER2 human epidermal growth factor receptor-2
Of the 107 HER2-positive patients, 36 were treated with trastuzumab (mostly in the City of Hope series); the majority did not receive trastuzumab because they were diagnosed before 2006. Table 3 shows the response to treatment for patients classified as HER2-type, for HER2-positive patients classified as Luminal-type, and for all HER2-positive patients. The pCR rate for HER2-positive breast cancer was 42 % for patients who did not receive trastuzumab, which is very similar to the pCR rate in patients treated with trastuzumab (47 %). However, the difference in the pCR rate for patients with HER2-type disease as identified by molecular subtyping was substantial, although not significantly different, with a pCR rate of 41 % in patients treated without trastuzumab versus 62 % in those treated with trastuzumab.
Discussion
The results of this analysis of patients from four independent neoadjuvant trials show that Luminal A-type (BluePrint Luminal-type/MammaPrint Low Risk) patients had low pCR rates but a good prognosis, with excellent DMFS at 5 years and little or no benefit from chemotherapy. MammaPrint enables subdivision of the Luminal group into two types, Luminal A and B, which cannot be achieved with standard pathology. The Basal-type subgroup with residual disease had the worst prognosis and the lowest 5-year DMFS rate (54 % for Basal-type breast cancer patients with residual disease). Patients with a pCR and HER2-type disease had a similar 5-year DMFS rate to those classified as HER2-positive by IHC/FISH (91 and 88 %, respectively). Remarkably, 43 % of HER2-positive patients were classified as Luminal-type by molecular subtyping; these Luminal-type/HER2-positive IHC/FISH patients had a dominant Luminal pathway despite being classified as HER2-positive by IHC/FISH assessment.
Not all IHC/FISH HER2-positive patients received neoadjuvant trastuzumab. A subgroup analysis of the trastuzumab-treated patients provides an indication that molecular subtyping is potentially a more accurate method to predict the response to trastuzumab than the classical IHC/FISH determination: 62 % of BluePrint HER2-type patients had a pCR compared with only 47 % of IHC/FISH HER2-positive patients. The data generated with molecular profiling are therefore more precise in predicting a pCR. This is in line with the results of a recent pooled analysis of a large cohort of patients using traditional IHC/FISH testing, which showed that the pCR rate is low in HER2-positive/HR-positive patients and not a suitable surrogate endpoint for prognosis in these patients [11]. In contrast, in Luminal B/HER2-negative, HER2-positive (non-Luminal), or basal (triple-negative) patients, pCR is a reliable surrogate endpoint and a good measure for chemosensitivity [11].
Our most important finding is the identification of the Luminal A-type subgroup (BluePrint Luminal-type/MammaPrint Low Risk): 90 of 437 (21 %) patients were classified as Luminal A-type. Luminal A-type patients, who are not identified by assessment using IHC/FISH for ER, PR, HER2, had a DMFS rate of 93 % at 5 years and showed little if any benefit from chemotherapy (the pCR rate was only 6 % in this group). The identification of this group could lead to improved treatment, with patients being able to avoid chemotherapy and to receive preoperative and adjuvant endocrine therapy alone. This analysis shows that molecular subtyping using BluePrint and MammaPrint has treatment implications for a substantial proportion of patients who are currently selected for neoadjuvant chemotherapy treatment based on IHC/FISH assessment. Patients in the Luminal B-type group had outcomes that were in line with those of patients assessed as HR-positive by IHC, i.e., a limited proportion of patients with a complete response to chemotherapy, as was also seen in other neoadjuvant studies, and pCR being a reliable measure of chemosensitivity.
As in previous studies [9, 31], our results demonstrate that patients with different breast cancer subtypes have different clinical outcomes with neoadjuvant chemotherapy (including trastuzumab in 34 % of IHC/FISH HER2-positive patients). Our results also confirm the importance of classifying breast cancer into molecular subtypes to select the appropriate patients who will benefit from neoadjuvant chemotherapy. However, we should point out that our analysis was not prospective and was performed on data from trials involving different institutions, chemotherapy regimens, and definitions of pCR. A recent meta-analysis from the German Breast Group also showed different clinical outcomes with neoadjuvant chemotherapy according to the breast cancer subtype classified by IHC/FISH [11].
In summary, compared with IHC/FISH, molecular subtyping (e.g., using BluePrint and MammaPrint) leads to a more precise classification of patients with ESBC and a better correlation with long-term clinical treatment outcomes. Molecular subtyping leads to the identification of a substantial group of patients with Luminal A-type disease for whom the pCR provides little prognostic information, who have excellent survival irrespective of chemotherapy, and may therefore not need chemotherapy treatment.
Abbreviations
- ACAC:
-
Doxorubicin, cyclophosphamide, carboplatin, and nab-paclitaxel
- DMFS:
-
Distant metastases-free survival
- ER:
-
Estrogen receptor
- ESBC:
-
Early-stage breast cancer
- FDA:
-
Food and drug administration
- FISH:
-
Fluorescence in situ hybridization
- HER2:
-
Human epidermal growth factor receptor-2
- HR:
-
Hormone receptor
- IHC:
-
Immunohistochemistry
- LABC:
-
Locally advanced breast cancer
- pCR:
-
Pathologic complete response
- PR:
-
Progesterone receptor
- TAC:
-
Docetaxel, doxorubicin, and cyclophosphamide
References
Aigner J, Schneeweiss A, Sohn C, Marmé F (2011) The role of neoadjuvant chemotherapy in the management of primary breast cancer. Minerva Ginecol 63:261–274
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, Karn T, Liedtke C, Mauri D, Rouzier R, Ruckhaeberle E, Semiglazov V, Symmans WF, Tutt A, Pusztai L (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19:1508–1516
Mieog JS, van de Velde CJ (2009) Neoadjuvant chemotherapy for early breast cancer. Expert Opin Pharmacother 10:1423–1434
Hortobagyi GN (2012) Neoadjuvant systemic therapy: promising experimental model, or improved standard of care? San Antonio breast cancer symposium, William L. McGuire Memorial Lecture
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Glück S, Ross JS, Royce M, McKenna EF Jr, Perou CM, Avisar E, Wu L (2012) TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat 132:781–791
Parker JS, Prat A, Cheang MCU, Lenburg ME, Paik S, Perou CM (2009) Breast cancer molecular subtypes predict response to anthracycline/taxane-based chemotherapy. San Antonio breast cancer symposium, abstract 2019
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678–5685
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. New Engl J Med 366:2438–2441
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:188–194
Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94:1189–1200
Food and Drug Administration Guidance for Industry (2012) Pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf. Accessed 2 Aug 2012
Perlmutter J, Axler S, Bass CC, Beckwith BJ, Bonoff A, Brain S, Delapine M, Devine M, Frank E, Fraser V, Gallece M, Geoghegan C, Hamade H, Heditsian D, Hirschhorn B, Kandell S, Laxague D, Lestage B, Lyzen M, Madden D, Mertz SA, Parker BJ, Roach N, Sauers N, Vincent L, Waddell D, Wetzel M, Wright K (2012) Advocates’ perspective: neoadjuvant chemotherapy for breast cancer. J Clin Oncol 30:4586–4588
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48:3342–3354
Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, Hudis C, Gray JW, Perou C, Yau C, Livasy C, Krontiras H, Montgomery L, Tripathy D, Lehman C, Liu MC, Olopade OI, Rugo HS, Carpenter JT, Dressler L, Chhieng D, Singh B, Mies C, Rabban J, Chen YY, Giri D, van ‘t Veer L, Hylton N (2012) Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol 30:3242–3249
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, NeoALTTO Study Team (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–640
Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Zujewski JA, Justice R, Loibl S, Wickerham L, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas E, Bergh J, Semiglazov V, Prowell T, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching P, Swain SM, Slaets L, Tang S, Gerber B, Geyer C, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Mincwitz G (2012) Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC). Cancer Res 72(24 Suppl):S1–S11
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. New Engl J Med 360:790–800
Krijgsman O, Roepman P, Zwart W, roll JS, Tian S, de Snoo FA, Bender RA, Bernards R, Glas AM (2012) A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat 133:37–47
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160–1167
Prat A, Ellis MJ, Perou CM (2011) Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol 9:48–57
Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7:e1000279
Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MC, Mardis ER, Perou CM, Bernard PS, Ellis MJ (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16:5222–5232
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, Panel members (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22:1736–1747
Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, Mariani G, Rodriguez J, Carcangiu M, Watson D, Valagussa P, Rouzier R, Symmans WF, Ross JS, Hortobagyi GN, Pusztai L, Shak S (2005) Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol 23:7265–7277
Straver ME, Glas AM, Hannemann J, Wesseling J, van de Vijver MJ, Rutgers EJ, Vrancken Peeters MJ, van Tinteren H, Van’t Veer LJ, Rodenhuis S (2010) The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 119:551–558
Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, DeMichele A, Gray JW, Conway-Dorsey K, Lenburg ME, Buxton MB, Davis SE, van’t Veer LJ, Hudis C, Chin K, Wolf D, Krontiras H, Montgomery L, Tripathy D, Lehman C, Liu MC, Olopade OI, Rugo HS, Carpenter JT, Livasy C, Dressler L, Chhieng D, Singh B, Mies C, Rabban J, Chen YY, Giri D, Au A, Hylton N, I-SPY 1 TRIAL Investigators (2012) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 132:1049–1062
Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, Booser D, Theriault RL, Buzdar AU, Dempsey PJ, Rouzier R, Sneige N, Ross JS, Vidaurre T, Gómez HL, Hortobagyi GN, Pusztai L (2006) Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol 24:4236–4244
Iwamoto T, Lee JS, Bianchini G, Hubbard RE, Young E, Matsuoka J, Kim SB, Symmans WF, Hortobagyi GN, Pusztai L (2011) First generation prognostic gene signatures for breast cancer predict both survival and chemotherapy sensitivity and identify overlapping patient populations. Breast Cancer Res Treat 130:155–164
Somlo G, Frankel PH, Vora L, Lau S, Luu TH, Kruper L, Yim J, Yen Y, de Snoo F, Bender RA (2010) Gene signatures as predictors of response to neoadjuvant chemotherapy (NCT) with docetaxel, doxorubicin, cyclophosphamide (TAC), or AC and nab-paclitaxel (nab-P) and carboplatin ± trastuzumab in patients (pts) with stage II–III and inflammatory breast cancer (IBC). J Clin Oncol 28(Suppl):540
National Cancer Institute. (2012) I-SPY home page. http://ispy.nci.nih.gov. Accessed 2 Aug 2012
Acknowledgments
This study was funded by an unrestricted grant from Agendia. The City of Hope trial was supported by American Bioscience (now Celgene). The authors would like to thank Kevin De-Voy (freelance medical writer funded by Agendia) for writing support.
Conflict of interest
SG has received research support from and been an advisory board member for Agendia and Genomic Health Inc. FdS, JP, and LS-S are employees of Agendia. GS had received grants from the National Institutes of Health and has been an advisory board member and speaker for Celgene and Genentech. George Somlo has received research support from Celgene.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glück, S., de Snoo, F., Peeters, J. et al. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat 139, 759–767 (2013). https://doi.org/10.1007/s10549-013-2572-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2572-4