Abstract
Ionizing radiation is a known cause of myeloid leukemia, but it is not known whether therapeutic doses for breast cancer (BC) pose an increased risk. We hypothesized that BC radiation treatment is associated with increased risk of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) as seen in a previously conducted study. We used 2001–2009 Surveillance, Epidemiology, and End Results (SEER) database records to identify a cohort of women with first primary stage 0 BC who were treated with radiation, a group which is not treated with chemotherapy. We identified subsequent MDS/AML diagnoses in the cohort using SEER to query appropriate ICD-O-3 codes. We compared observed MDS/AML rates in the BC cohort to expected rates, estimated as first primary MDS/AML in the entire female population, and calculated observed/expected rate ratios with 95 % confidence intervals (CI). Overall, a very small number of cases of MDS/AML occurred in this cohort with 22 observed cases versus 9.4 expected cases using national incidence data. We estimated an increased risk of 2.34 for MDS/AML in stage 0 BC cases treated with radiation compared to the general population (95 % CI 1.49, 3.46, p < 0.001). The age adjusted relative risk is 1.46, (95 % CI 0.93, 2.16, p = 0.08). Our results suggest that radiation treatment for BC is associated with an increased risk of MDS/AML and affects a very small number of patients.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Ionizing radiation has been recognized as a potent carcinogen. Radiation exposure has been linked to many forms of cancer with leukemia being particularly sensitive to induction, especially at younger ages [1, 2]. Only a few cohort studies of women with secondary cancer post breast cancer (BC) treatment have been conducted which include radiation treatment patients as a separate group [3, 4]. In an earlier study of an institutional cohort, we observed an increased risk of myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) among female BC patients treated with radiation and no chemotherapy, suggesting a possible role of radiation in the pathogenesis of these second malignancies [5]. In an analysis of the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) data for the incidence of second cancers in female BC patients, a significant increased risk of leukemia related to radiation therapy was observed by Yu et al. [6]. In a review of SEER data for incidence of AML post treatment with radiation for prostate cancer, an increased hazard of leukemia was observed among men treated with external beam radiation therapy, but not among men treated with brachytherapy [7].
In light of the addition of MDS as a reportable disease to the SEER database in 2001, we used the National Cancer Institute SEER program to identify BC patients treated with radiation to calculate the risk of MDS and AML combined compared to women without BC stratified by age. As many cases of MDS will transform into AML and MDS has been identified to be associated with a previous cancer diagnosis and radiation treatment, it is a logical step to include both of these entities in the analysis of secondary leukemia post BC treatment with radiation [8]. Given the treatment reporting limitations of a large national registry database, we restricted our analysis to stage 0 BC cases, a group that is not treated with chemotherapy. Our study improves upon previously done studies including our own of secondary leukemia post BC treatment by using a much larger database (SEER), expanding the myeloid cancer outcomes to include MDS, and restricting the analysis to the subgroup receiving radiation only.
Methods
Data sources
Data from the eighteen tumor registries participating in the SEER program were studied. SEER is a national registry containing population-based incidence data on cancer cases covering approximately 28 % of the United States population [9]. Data collected includes demographic data such as gender and age and disease specific information including diagnosis date, International Classification of Diseases for Oncology (ICD-O-3) coded diagnosis, stage at diagnosis, surgery, radiation treatment, and survival status. Multiple primary malignancies are recorded for an individual patient for as many diagnoses that occur when the person resides in one of the participating SEER registry sites [10]. We used SEERstat version 7.0.9 software to identify cancer cases and estimate rates from SEER 18 for years 2001–2009 [11].
We included all women with stage 0 BC diagnosed in 2001–2009 (the years after which MDS became a reportable malignancy) with recorded radiation treatment. Treatment with radiation for stage 0 BC is supported by the National Cancer Care Network (NCCN) guidelines Version 3.2012 which indicates lumpectomy with radiation, total mastectomy with or without radiation, or lumpectomy without radiation as the three possible treatment recommendations [12]. Chemotherapy is not indicated or recommended for stage 0 cases also known as ductal carcinoma in situ (DCIS). Cases of lobular carcinoma in situ (LCIS) stage 0 BC were excluded as they are usually treated with excisional biopsy (surgery) alone.
We followed BC cases from 2001–2009 for second primary diagnosis of MDS (ICD-O-3 codes 9980, 9982–9987, 9989) or AML (ICD-O-3 codes 9840, 9861, 9866–9867, 9871–9874, 9895–9897, 9910, 9920). We calculated the person years of followup for each BC patient as the length of time from BC diagnosis to (1) diagnosis of second primary MDS/AML; (2) diagnosis of a different second primary cancer; or (3) death, whichever occurred first.
Only BC cases with at least 3 months of followup were included in statistical analyses to avoid inclusion of MDS/AML cases that may have been simultaneously diagnosed with the BC and are therefore unlikely to be associated with radiation treatment. We included the shorter time frame to capture all cases that may be treatment related.
Analysis
Background rates of MDS/AML were calculated for age groups 15–65+ and age 40–65+. In addition to the crude rates from the US standard 2000 population, stratification-based age adjustment was done to standardize incidence rates of expected cases to the reference population of women with stage 0 BC (Table 1). The second age grouping (40–65+) was used to limit the comparison to the age at which women are likely to participate in mammography screening programs as stage 0 BC (DCIS) is almost exclusively a mammography detected disease and age 40 is the age at which mammography screening recommendations begin in the United States [13, 14]. These steps were taken as 2.5 % of SEER stage 0 cases are 15–39, but the SEER US standard population is 53 % age 15–39. Thus, the adjusted background rates are based on cases representative of a population with the same age distribution as our cohort of women with stage 0 BC.
Rates of incident MDS/AML in the cohort of stage 0 BC cases with reported radiation treatment were compared to background rates of MDS/AML reported in SEER as a first primary cancer in women ages 15–64 and age 65+ and ages 40–65 and 65+ in 2001–2009. Observed incidence rates were calculated by the number of events divided by the total number of person years of followup. Rate ratios and 95 % confidence intervals for rates and rate ratios were obtained from Poisson regression [15].
Results
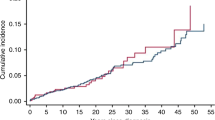
From the 18 SEER regions 2001–2009, there were 79,756 stage 0 BC cases with at least 3 months of followup with radiation treatment reported for 36,191 (45.4 %) of these cases (Table 1). The age range for the cases (n = 22) is from age 50–84 years and for the total DCIS BC group age 15–85+ years. Age breakdown of the stage 0 BC cohort with radiation treatment and subsequent MDS/AML incidence was 31.8 % age 50–64 (n = 7) and 68.2 % age 65–84 (n = 15). The majority of the 22 MDS/AML post BC cases had their BC diagnosis in 2001–2003 (n = 16). The age composition of the total stage 0 BC group was 70.8 % age 15–64 and 29.2 % age 65–85+. Followup status for all cases was 2 % = died any cause, 92 % = alive, 0.06 % = myeloid event, and 6 % = other cancer event (Table 1).
The 22 cases of MDS/AML post BC treatment with radiation had mean followup of 49.66 months with a range of 3.04–87.47 months. Of the 36,191 stage 0 BC cases with radiation treatment reported to SEER 2001–2009, mean followup was 49.77 months with range 3.01–108.16 months.
Overall, a very small number of cases of MDS/AML occurred in this population with 22 observed cases versus 9.4 expected (Table 2). We estimated an unadjusted increased overall risk of MDS/AML in the stage 0 BC cases treated with radiation compared to the general population (RR = 2.34, 95 % CI 1.49, 3.46, p value < 0.001). The unadjusted relative risk was higher for stage 0 BC cases <65 years of age (RR = 2.44, 95 % CI 1.05, 4.72, p value = 0.02) than for stage 0 cases 65 years and older (RR = 1.27, 95 % CI 0.73, 2.02, p value = 0.4). When the rates were age adjusted the significance for the age 15–65+ age group was at the 0.08 level with an increased risk of 1.46 (95 % CI 0.93, 2.16) and when the age group was restricted to age 40–65+ the significance was p = 0.06 with an increased risk of 1.50 (95 % CI 0.96, 2.22).
Discussion
Using the SEER database, we observed a small, but significant, increased risk of second primary MDS/AML incidence among women with stage 0 BC who were treated with surgery and radiation. Owing to small numbers in the subcategories of age, this significance could not be further defined by age. An increased risk in younger women has been reported by Martin et al. [16] in their analysis of SEER data for second cancers including AML following a diagnosis of stage I–III BC. Yu et al. [6] observed a rate of 1.8 increased risk of second leukemia post radiation treatment for BC (95 % CI 1.2–2.8) was obtained by an inception cohort method and risk ratios calculated comparing patients with radiation treatment to those without. In the 1985 report of the National Surgical Adjuvant Breast and Bowel Project experience by Fisher et al. [17], a significant increased risk of leukemia post BC treatment with radiation was also observed.
In our previous institutional cohort study, we observed a threefold increased risk of MDS/AML in BC patients who received radiation treatment, but no chemotherapy (N = 7/2764, RR = 3.32, 95 % CI 1.42, 6.45) [5]. That study differed from the current one as our analysis included stage 0 and stage I BC cases with radiation treatment only. By reviewing data from the SEER registry database, we have used a much larger database, restricted our analysis to stage 0 cases eliminating the possibility of confounding from other treatment (chemotherapy) and added MDS which has not been previously studied in relationship to BC treatment with radiation. However, not including stage I cases treated only with radiation post surgery, we reduced the number of potentially exposed patients. SEER does not include reporting of chemotherapy so we were unable to identify Stage I patients treated with radiation only. SEER reporting of radiation treatment is 72–79 % accurate when compared to patient report or medical record of radiation treatment receipt [18, 19]. Given a probability of underascertainment, but no evidence from published studies of overascertainment using SEER data, radiation treatment as an exposure for subsequent blood malignancies is supported.
Our findings have significant implications for patient treatment with radiation post surgery given the number of new cases of low risk BCs diagnosed every year by screening mammography. Mammography detected low grade disease has very little mortality risk with a concomitant increase in survivorship. While the increased risk of AML/MDS reported here is quite low, it is nevertheless important to limit the use of potentially toxic therapy as much as is prudent in the treatment of this disease. In a study of patients with invasive BC, Hughes et al. [20] have found that in older women tamoxifen alone can be an effective alternative to tamoxifen plus radiation treatment. Hughes et al. [21] found in a trial of the Eastern Cooperative Oncology Group that patients with low to intermediate grade DCIS and adequate excisional margins had an acceptably low rate of recurrence without radiation post lumpectomy. Hopefully, newer techniques such as gene array analysis will allow identification of patients who do not require radiation therapy after surgery [22].
Similarly, for those who do require radiation, it is possible that newer radiation techniques such as intensity-modulated radiation therapy (IMRT) or MammoSite radiation will change radiation dosing in a way that will reduce the risk of MDS/AML [23, 24]. At this point in time, however, there is concern that the risk of BC relapse with such techniques may be increased over traditional techniques and we have no data on risk of malignancy with the newer treatments [25].
Although the risk of MDS/AML post radiation treatment remains very small, there is a consistent increased number of MDS/AML cases post radiation treatment for stage 0 BC that may be age dependent. It will be important to continue tracking risk of second cancers and MDS/AML in particular for risk associated with radiation treatment. These results do not dispute the proven benefits of radiation treatment for preventing local recurrence. However, we should continue efforts to develop treatments for BC that provide the highest possible cure rates with the least systemic toxicity. Limiting radiation exposure to the least amount necessary and utilizing algorithms to individualize treatment plans specific to the patient should be part of that effort.
References
Li CI, Nishi N, McDougall JA, Semmens EO, Sugiyama H, Soda M et al (2010) Relationship between radiation exposure and risk of second primary cancers among atomic bomb survivors. Cancer Res 70(18):7187–7198
Wakeford R (2004) The cancer epidemiology of radiation. Oncogene 23(38):6404–6428
Curtis R, Boice J, Stovall M, Bernstein L, Greenburg RS, Flannery JT, Schwartz AG, Weyer P, Moloney WC, Hoover RN (1992) Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 326:1745–1751
Renella R, Verkooijen HM, Fioretta G, Vlastos G, Kurtz J, Sappino AP, Schafer P, Neyroud-Casper I, Bouchardy C (2006) Increased risk of acute myeloid leukaemia after treatment for breast cancer. Breast 15:614–619
Kaplan HG, Malmgren JA, Atwood MK (2011) Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer 11:260
Yu G-P, Schantz SP, Neugut AI, Zhang Z-F (2006) Incidences and trends of second cancers in female breast cancer patients: a fixed inception cohort-based analysis (United States). Cancer Causes Control 17:411–420
Ojha RP, Fischbach LA, Zhou Y, Felini MJ, Singh KP, Thertulien R (2010) Acute myeloid leukemia incidence following radiation therapy for localized or locally advanced prostate adenocarcinoma. Cancer Epidemiol 34:274–278
De Roos AJ, Deeg HJ, Davis S (2007) A population-based study of survival in patients with secondary myelodysplastic syndromes (MDS): impact of type and treatment of primary cancers. Cancer Causes Control 18:1199–1208
http://seer.cancer.gov/about/overview.html. Accessed 13 July 2012
http://www.who.int/classifications/icd/en/. Accessed 13 July 2012
http://seer.cancer.gov/seerstat/software/. Accessed 12 Sept 2012
NCCN guidelines version 3.2012 © National Comprehensive Cancer Network, Inc. 2012. Accessed 9 Sept 2012
Malmgren JA, Atwood MK, Kaplan HG (2008) Increase in mammography detected breast cancer over time at a community based regional cancer center: a longitudinal cohort study 1990–2005. BMC Cancer 8:131. doi:10.1186/1471-2407-9-131
American Cancer Society Breast Cancer Screening Guidelines. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Accessed 27 Nov 2012
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman and Hall, London
Martin MG, Welch JS, Luo J, Ellis MJ et al (2009) Therapy related acute myeloid leukemia in breast cancer survivors, a population based study. Breast Cancer Res Treat 118:593–598
Fisher B, Rockette H, Fisher ER, Wickerham L et al (1985) Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol 3(12):1640–1658
Malin JL, Kahn KL, Adams J et al (2002) Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst 94(11):835–844
Jagsi R, Abrahmse P, Hawley ST et al (2012) Underascertainment of radiotherapy receipt in surveillance, epidemiology, and end results registry data. Cancer 118:333–341
Hughes KS, Schnaper LA, Cirrincione C, Berry DA et al (2010) Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. J Clin Oncol 28(15):507
Hughes LL, Wang M, Page DL, Gray R et al (2009) Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27(32):5319–5324
Badve S, Gray R, Baehner F, Solin L et al (2012) Correlation between the DCIS score and traditional clinicopathologic features in the prospectively designed E5194 clinical validation study. J Clin Oncol 30(Supp 1):1005
Woo TCS, Pignol J-P, Rakovitch E, Vu T et al (2006) Body radiation exposure in breast cancer radiotherapy: impact of breast IMRT and virtual wedge compensation techniques. Int J Radiat Oncol 65(1):52–58
Streeter OE, Vicini FA, Keisch M, MA Astrahan et al (2003) MammoSite radiation therapy system. Breast 12(6):491–496
Smith GL, Xu Y, Buchholz TA, Giordano SH et al (2012) Association between treatment with brachytherapy versus whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. J Am Med Assoc 307(17):1827–1837
Acknowledgments
Supported by the Kaplan Cancer Research Fund.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaplan, H., Malmgren, J. & De Roos, A.J. Risk of myelodysplastic syndrome and acute myeloid leukemia post radiation treatment for breast cancer: a population-based study. Breast Cancer Res Treat 137, 863–867 (2013). https://doi.org/10.1007/s10549-012-2386-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2386-9