Abstract
Aspects of reproductive history are among the most well-established breast cancer risk factors. However, relatively little is known about how they influence risk of different molecular subtypes of breast cancer, particularly among younger women. Using data from a population-based case–control study of women 20–44 years of age, we assessed the relationships between various reproductive factors and risk of estrogen receptor positive (ER+), triple-negative, and HER2-overexpressing breast cancers. Detailed reproductive histories were obtained through structured interviewer administered in-person questionnaires. Reproductive histories among control women (n = 941) were compared to those of ER+ cases (n = 781), triple-negative cases (n = 180), and HER2-overexpressing cases (n = 60) using polytomous logistic regression. Age at menarche, parity, and number of full-term pregnancies were similarly associated with risk of all three breast cancer subtypes. In contrast, age at first live birth, the interval between age at menarche and age at first birth, and breastfeeding were inversely associated with risk of triple-negative breast cancer (P values for trend 0.002, 0.006 and 0.018, respectively), but were not associated with risk of ER+ or HER2-overexpressing cancers. A strong inverse association between breastfeeding and risk of triple-negative breast cancer has now been consistently observed across numerous studies, and at present it is the most well-established protective factor for this aggressive and lethal form of breast cancer. Further studies clarifying the biological mechanisms underlying this relationship and confirming our results with respect to age at first birth and the interval between age at menarche and age at first birth are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproductive factors are among the earliest and most well-established breast cancer risk factors. With respect to premenopausal breast cancer in particular, there is compelling evidence from pooled analyses that the risk of premenopausal breast cancer is reduced 9 % for each year of age menarche is postponed, is increased by 5 % for each additional year age at first birth is delayed [1], and is reduced by 4 % with each additional 12 months of breastfeeding [2]. However, almost all studies evaluating relationships between reproductive factors and premenopausal breast cancer risk have grouped all breast cancers together with few stratifying results according to tumor subtypes. The identification and validation of distinct molecular subtypes of breast cancer based on patterns of gene expression have shifted that how we approach this complex disease [3, 4]. The most common subtypes are estrogen receptor-positive (comprising the luminal A and luminal B subtypes), while two of the more aggressive subtypes, which carry comparatively poorer prognoses, are triple-negative tumors [they lack estrogen receptor (ER), progesterone receptor (PR), and HER2-neu (HER2) expression and the majority of them have the so-called basal-like phenotype] and HER2-overexpressing tumors (ER−/HER2+) [5, 6]. The unique molecular characteristics of the different subtypes along with the considerable variability in their prognoses suggest that they likely have unique etiologies.
Only a handful of published studies have evaluated how reproductive factors may be differentially associated with risk of different molecular subtypes of breast cancer. Only one has presented results specifically focused on young women (35–44 years of age) [7], and the remainder either did not stratify results by age or menopausal status [8–10], or they only included postmenopausal women [11, 12]. Studies focused on young women are of particular relevance because triple-negative and HER2-overexpressing tumors both account for higher proportions of cases among premenopausal women than they do among postmenopausal women [9], and it is well established that the strength and direction of associations between reproductive factors and premenopausal versus postmenopausal breast cancer vary [1, 13]. Here, we present results from a study focused on quantifying the relationships between reproductive factors and risk of different molecular subtypes of breast cancer among young women 20–44 years of age.
Methods
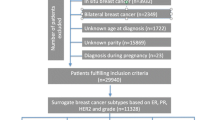
The design and overall methods employed in this study have been previously published [14]. Briefly, we conducted a population-based case–control study where all women 20–44 years of age diagnosed with invasive breast cancer between June 2004 and June 2010 in the three county Seattle–Puget Sound metropolitan area were eligible as cases. These patients were identified through our local population-based cancer registry. Of the 1,359 eligible cases identified, 1,056 (78 %) were interviewed. Data on tumor characteristics were obtained from the cancer registry and from a centralized review of pathology reports including data on ER, PR, and HER2 status. ER and PR positivity were defined as positive staining of ≥1 % of cells and negativity as 0 or <1 % positive staining of cells. HER2 positivity was based on an immunohistochemistry (IHC) score of 3+ and/or a FISH-positive result, and negativity was defined as an IHC score of 0 or 1+ and/or a FISH-negative result. This information was used to group cases into three groups approximating the different molecular subtypes of breast cancer: ER+ (approximating the luminal subtypes), ER−/PR−/HER2− (this triple-negative group approximates the basal-like subtype), and ER−/HER2+ (approximating the HER2-overexpressing subtype). This approach has been used in several other studies focused on characterizing risk factors for different molecular subtypes of breast cancer [7, 8, 11, 12, 15–17]. The 28 cases for whom data on ER, PR, and/or HER2 status were missing could not be classified by subtype and were therefore excluded from all analyses.
A population-based control group, frequency matched within 5-year age groups to the cases, was identified and recruited using random digit dialing. We used a combination of list-assisted (purchased randomly generated telephone numbers) and Mitofsky–Waksberg (telephone numbers randomly generated ourselves using a clustering factor of 5) [18] random digit dialing methodologies. Of the 1,489 eligible controls identified, 943 (63 %) were interviewed.
Data collection
The study protocol was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, and written informed consent was obtained from all study subjects. Cases and controls were interviewed in-person and asked about a variety of exposures. In particular, comprehensive reproductive histories were obtained including details relating to each pregnancy participants ever had such as their age at each pregnancy, the pregnancy’s outcome, and if the pregnancy was a live birth their breastfeeding history. Using this information, we assessed the relationship between age at menarche, parity, number of live births, age at first live birth, age at last birth, and breastfeeding in relation to risks of ER+, triple-negative, and HER2-overexpressing breast cancers. In addition, we estimated risks associated with the time interval between age at menarche and age at first live birth. All our questions were limited to exposures that occurred before each participant’s reference date. The reference date used for each woman with breast cancer was her diagnosis date, and controls were assigned reference dates that reflected the distribution of reference dates among the cases. Data on one or more reproductive factors were missing for two controls and three cases. These participants were excluded from all analyses and thus our final analytic data set consisted of 941 control women, 781 ER+ cases, 184 triple-negative cases, and 60 HER2-overexpressing cases.
Statistical analysis
Polytomous logistic regression was used to simultaneously estimate the risks associated with a particular aspect of reproductive history in relation to each of the three breast cancer subtypes in comparison to controls within a single statistical model. These models calculated odds ratios (OR), which approximate relative risks, and their associated 95 % confidence intervals (CI) [19]. P values for trend where appropriate were calculated by treating categorical variables as ordered continuous variables. P values comparing risks across case types were performed in analyses that excluded the control group and used the ER+ case group as the reference category. All analyses were adjusted for age (in 5-year groups) and reference year (continuous) as controls were matched to cases on these factors. We a priori adjusted our analysis of number of live births for age at first live birth, our analysis of the interval between age at menarche and age at first live birth for number of live births, and our analysis of breastfeeding for age at first live birth and number of live births. None of the other potential confounders listed in Table 1 changed our risk estimates by more than 10 % when individually assessed and so none was adjusted for in our final statistical models. In particular, first-degree family history of breast cancer was neither a confounder nor an effect modifier (based on likelihood ratio testing). All analyses were conducted using Stata/SE version 11.2 (StataCorp LP, College Station, TX, USA).
Results
Compared to controls, ER+ cases were somewhat older and more likely to be Asian/Pacific Islander, and triple-negative and HER2-overexpressing cases were somewhat younger and more likely to be African American (Table 1). Triple-negative cases were also somewhat more likely to be less highly educated, to have higher annual household incomes, and to have used oral contraceptives compared to controls.
Age at menarche was not statistically significantly related to risk of any of the three breast cancer subtypes (Table 2). Parity was associated with a 30 % reduction in risk of both ER+ (95 % CI 0.5–0.8) and triple-negative (95 % CI 0.5–1.0) breast cancer, but not with risk of HER2-overexpressing disease (OR = 1.1, 95 % CI 0.6–2.2). Increasing number of live births was similarly associated with reduced risks of all three breast cancer subtypes although the only statistically significant association was in relation to ER+ breast cancer (P for trend = 0.015). While there was some suggestion that increasing age at first birth was associated with reduced risks of all three breast cancer subtypes, this relationship was only statistically significant for triple-negative breast cancer (P for trend = 0.002). Furthermore, the association with triple-negative disease was statistically different from the one between age at first live birth and risk of ER+ breast cancer (P value for comparison between these case groups = 0.034). The interval between age at menarche and age at first live birth was inversely related to risk of triple-negative breast cancer (P for trend = 0.006) but not to risk of ER+ (P value for the ER+ vs. triple-negative cases = 0.051). Finally, breastfeeding was not associated with risk of either ER+ or HER2-overexpressing breast cancer, but was associated with a reduced risk of triple-negative disease. This association was statistically different from the relationship between breastfeeding and ER+ breast cancer (P values for difference 0.009).
Discussion
The most notable differences in risk we observed by breast cancer subtype were with age at first live birth, the interval between age at menarche and age at first live birth, and breastfeeding. With respect to age at first birth, a pooled analysis of much of the world’s literature suggests that risk of premenopausal breast cancer is increased by 5 % for each additional year age at first birth is delayed [1]. The results presented here suggest that increasing age at first birth was associated with a reduced risk of triple-negative breast cancer that was stronger in magnitude than the non-statistically significant reduced risks observed with respect to both ER+ and HER2-overexpressing breast cancers. Only six prior studies have assessed the relationship between age at first birth and breast cancer risk according to molecular subtype [7–12]. Four found that age at first birth was not related to risk of any of these three subtypes of breast cancer [7, 10–12], including the only previous study to present data specific to younger women (35–44 years of age) [7]. The other two studies found that age at first live birth was positively associated with risk of luminal A breast cancer, but was not associated with risk of any other breast cancer subtype [8, 9]. Of note, in all these studies there were relatively few women with later ages at first birth. In three studies, 90 % [11], 92 % [12], and 79 %[7] of the controls, respectively had an age at first birth of <30, in another 76 % had an age at first birth of <26 [9], and in two others the mean ages at first birth were 23.6 [10] and 22.5 [8] years. In contrast, in our study which was more recently conducted, only 59 % of parous controls had their first live birth at ≤30 years of age and the mean age at first birth was 27.8 years. It is possible that the comparatively smaller proportions of women with later ages at first birth in previous studies may have limited their statistical power.
The interval between age at menarche and age at first live birth is a metric that has only been assessed by a handful of studies, but it is of interest because it represents the period of time over which postpubertal breast tissue is relatively undifferentiated and potentially more susceptible to carcinogenic insults until the differentiation induced by pregnancy confers a long-term reduction in breast cancer risk occurs. Prior studies suggest that this interval is positively associated with breast cancer risk, but none has assessed risk according to joint ER/PR/HER2 status [20–23]. The novel finding here is that this interval was inversely associated with risk of triple-negative breast cancer. There was also a similar non-statistically significant suggestion that this interval was inversely associated with risk of HER2-overexpressing breast cancer, but this analysis was hampered by the comparatively few number of HER2-overexpressing cases included. These relationships with triple-negative and HER2-overexpressing breast cancers are in the opposite direction of what has been observed for breast cancer overall in prior studies, but as this is the first report of these relationships these findings require confirmation.
A pooled analysis of 47 studies estimated that breast cancer risk is reduced by 4 % with each additional 12 months of breastfeeding [2]. Here, we observed that breast feeding is associated with a substantial reduction in risk of triple-negative breast cancer only, as it did not influence risk of either ER+ or HER2-overexpressing breast cancer. Five more recently published studies have evaluated this relationship by breast cancer subtype [7–9, 11, 12]. Three found that breastfeeding was statistically significantly associated with a reduced risk of triple-negative or basal-like breast cancer but was not associated with risk of ER+ or luminal A breast cancer [8, 9, 12], one study restricted to only postmenopausal women observed similar relationships though the trend for triple-negative breast cancer was not statistically significant [11], and one study found that breastfeeding was associated with reduced risks of similar magnitudes for both triple-negative and luminal A type breast cancers among young women 35–44 years of age [7]. The magnitude and direction of the relationship between breastfeeding and triple-negative breast cancer and the lack of an association with ER+ breast cancer observed here are consistent with the results of the majority of the studies that have assessed these relationships. Our results therefore add to the growing body of evidence that breastfeeding may indeed confer a lower risk of triple-negative breast cancer, a finding that here is supported by both our case–control (P for trend in the case–control comparison = 0.02) and case–case comparisons (P for comparison to the ER+ case group = 0.01). With the addition of our results, at present breastfeeding is the most consistently identified factor to be differentially associated with risk of triple-negative breast cancer compared to the other major molecular subtypes of the disease.
The biological mechanisms through which a late age at first birth, a longer interval between age at menarche and age at first live birth, and breastfeeding could preferentially confer a lower risk of triple-negative or basal-like breast cancer are largely unknown. These exposures are thought to influence breast cancer risk through the structural changes and differentiation of terminal ductal lobular units in breast tissue they are related to, rather than to be due to hormonal effects [24, 25]. Parous women experience differentiation of breast tissue that nulliparous women never do, and breastfeeding results in even greater differentiation. Further studies are required to replicate our findings with respect to age at first birth and the interval between age at menarche and age at first live birth as the relationships observed here between these two exposures and risk of triple-negative breast cancer are in the opposite direction from what has been observed for breast cancer overall. Why these exposures would have an opposite effect on triple-negative tumors remains unknown making replication critical. Alternatively, given the remarkable consistency across diverse populations with respect to the relationship between breastfeeding and triple-negative breast cancer further mechanistic studies to better understand how breastfeeding may confer a lower risk of this specific subtype of breast cancer are warranted. Such studies are not only of etiologic interest but also point to new approaches to prevent this particular aggressive form of breast cancer.
The potential protective effects of age at first birth and breastfeeding in relation to triple-negative breast cancer could in part also explain some of the demographic differences in the occurrence of triple-negative breast cancers observed in the United States. Specifically, it has been well characterized that higher proportions of breast cancer diagnosed among African American women are triple-negative compared to proportions among white women [5, 8, 9, 26–29]. It has also been shown that African American women are more likely to have a younger age at first birth and to never have breastfed compared to white women. In one study of women <40 years of age, 78 % of African Americans and 59 % of whites had their first birth before age 26 (P for difference = 0.04) and 82 % of parous African American women never breastfed compared to 61 % of white women (P for difference = 0.01) [9]. There were too few African American women in the study conducted here to construct the models needed to formally assess the extent to which differences in these reproductive characteristics could account for the observed higher risks of triple-negative breast cancer that African American women experience. While further work is needed, these data do suggest that differences in risk factor distributions may be of equal or greater relevance than biological or genetic differences with respect to explaining the greater burden of triple-negative disease among African Americans.
There is inconsistency in the literature on the relationship between parity and risk of different breast cancer subtypes. Our observation that parous women have reduced risks of both ER+ and triple-negative breast cancer is consistent with the only two published studies presenting analyses restricted to women <45 years of age that have evaluated this relationship [7, 8]. In contrast, two studies, one including similar numbers of premenopausal and postmenopausal women and one of exclusively postmenopausal women, found that while parity was associated with a reduced risk of luminal A/ER+ breast cancer it was associated with an increased risk of triple-negative breast cancer [9, 11]. So while parity has been consistently associated with a reduced risk of ER+ breast cancer, its relationship to triple-negative breast cancer remains uncertain though it may vary according to menopausal status.
The primary limitation of this study relates to its case–control design. Recall bias is always a theoretical concern; however, this study was restricted to younger women and focused on noteworthy life events regarding timing of full-term pregnancies and breastfeeding that should be recalled similarly and accurately by both cases and controls. Selection bias is also a concern. Our response rates for cases and controls were reasonable, though response rates were higher among cases compared to controls. Further counteracting both potential recall and selection biases and enhancing the validity of our findings are the statistically significant results from our case–case comparisons as response rates did not vary by case type and recall of these exposures is unlikely to vary according to breast cancer subtype. Another limitation is that we lacked data on BRCA1 and BRCA2 mutation status. However, our results of first-degree family history were neither a confounder nor an effect modifier and the proportion of young breast cancer patients who carry BRCA1 or BRCA2 mutations is relatively low, ranging 1.3–6.8 and 2.0–4.0 %, respectively, based on the results of prior population-based studies [30–33].
There is a small but growing body of literature characterizing differences in the relationships between established breast cancer risk factors and risk of different molecular subtypes of breast cancer. The risk factor that has most consistently emerged thus far as being differentially associated with risk according to subtype is breastfeeding, as now five out of six studies have shown that breastfeeding is more strongly associated with a reduced risk of triple-negative/basal-like breast cancer than it is with ER+/luminal A breast cancer. Adding to the potential validity and generalizability of this relationship is the fact that it has been observed across studies conducted in different regions of the United States and that have included disparate age ranges. With respect to other reproductive factors, the picture is less clear, but variations by age, menopausal status, and demographic factors may also be highly relevant. The studies published thus far have each included relatively few triple-negative/basal-like cases (ranging from 78 to 335 cases) limiting statistical power for stratified analyses. Given the comparatively poor prognoses of triple-negative/basal-like breast cancers compared to ER+/luminal breast cancers, additional studies further characterizing their etiologic differences are needed with the hope that they can inform novel subtype-specific prevention strategies. Additional work is also needed to characterize factors that influence the risk of HER2-overexpressing breast cancer as this somewhat rarer subtype also carries a relatively poor prognosis. This study is consistent with other studies in finding that no reproductive factor appears to be related either positively or negatively to risk of HER2-overexpressing disease.
References
Clavel-Chapelon F, Gerber M (2002) Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat 72:107–115
Collaborative Group on Hormonal Factors in Breast Cancer (2002) Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 360:187–195
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502
Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G (2006) Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol 37:1217–1226
Ma H, Wang Y, Sullivan-Halley J, Weiss L, Marchbanks PA, Spirtas R, Ursin G, Burkman RT, Simon MS, Malone KE, Strom BL, McDonald JA, Press MF, Bernstein L (2010) Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res 70:575–587
Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas TW, Bernstein JL (2011) Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat 130:587–597
Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM (2008) Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 109:123–139
Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, Garcia-Closas M (2007) Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev 16:439–443
Phipps AI, Chlebowski R, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, Li CI (2011) Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst 103:470–477
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI (2008) Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer 113:1521–1526
Clavel-Chapelon F (2002) Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer 86:723–727
Li CI, Beaber EF, Chen Tang MT, Porter PL, Daling JR, Malone KE (2012) Effect of depo-medroxyprogesterone acetate on breast cancer risk among women 20 to 44 years of age. Cancer Res 72:2028–2035
Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, Li CI (2010) Family history of breast cancer in first-degree relatives and triple-negative breast cancer risk. Breast Cancer Res Treat 126:671–678
Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Vitolins M, Kabat GC, Rohan TE, Li CI (2011) Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev 20:454–463
Phipps AI, Malone KE, Porter PL, Daling JR, Li CI (2008) Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev 17:2078–2086
Waksberg J (1978) Sampling methods for random digit dialing. J Am Stat Assoc 73:40
Begg CB, Gray R (1984) Calculation of polychotomous logistic regression parameters using individualized regressions. Biometrika 71:11–18
Andrieu N, Prevost T, Rohan TE, Luporsi E, Le MG, Gerber M, Zaridze DG, Lifanova Y, Renaud R, Lee HP, Duffy SW (2000) Variation in the interaction between familial and reproductive factors on the risk of breast cancer according to age, menopausal status, and degree of familiarity. Int J Epidemiol 29:214–223
Andrieu N, Smith T, Duffy S, Zaridze DG, Renaud R, Rohan T, Gerber M, Luporsi E, Le M, Lee HP, Lifanova Y, Day NE (1998) The effects of interaction between familial and reproductive factors on breast cancer risk: a combined analysis of seven case–control studies. Br J Cancer 77:1525–1536
Clavel-Chapelon F (2002) Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control 13:831–838
Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, Strom BL, Simon MS, Press MF, Ursin G, Burkman RT, Folger SG, Norman S, McDonald JA, Spirtas R (2008) Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol 167:230–239
Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ (1990) Comparative study of human and rat mammary tumorigenesis. Lab Invest 62:244–278
Russo J, Russo IH (1997) Role of differentiation in the pathogenesis and prevention of breast cancer. Endocr Relat Cancer 4:7–12
Brown M, Tsodikov A, Bauer KR, Parise CA, Caggiano V (2008) The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999–2004. Cancer 112:737–747
Lund MJ, Butler EN, Bumpers HL, Okoli J, Rizzo M, Hatchett N, Green VL, Brawley OW, Oprea-Ilies GM, Gabram SG (2008) High prevalence of triple-negative tumors in an urban cancer center. Cancer 113:608–615
Shinde SS, Forman MR, Kuerer HM, Yan K, Peintinger F, Hunt KK, Hortobagyi GN, Pusztai L, Symmans WF (2010) Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer 116:4933–4943
Stark A, Kapke A, Schultz D, Brown R, Linden M, Raju U (2008) Advanced stages and poorly differentiated grade are associated with an increased risk of HER2/neu positive breast carcinoma only in White women: findings from a prospective cohort study of African-American and White-American women. Breast Cancer Res Treat 107:405–414
Anglian Breast Cancer Study Group (2000) Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer 83:1301–1308
Loman N, Johannsson O, Kristoffersson U, Olsson H, Borg A (2001) Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J Natl Cancer Inst 93:1215–1223
Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, Marchbanks PA, Simon MS, McDonald JA, Norman SA, Strom BL, Burkman RT, Ursin G, Deapen D, Weiss LK, Folger S, Madeoy JJ, Friedrichsen DM, Suter NM, Humphrey MC, Spirtas R, Ostrander EA (2006) Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66:8297–8308
Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91:943–949
Acknowledgments
This study was funded by the National Cancer Institute (R01-CA105041 and ARRA supplement to R01-CA10541) and the Department of Defense Breast Cancer Research Program (W81XWH-05-1-0482). The authors also wish to acknowledge the substantial contributions of Kristine Wicklund, Christabel Fowler, and Anne Oswald in the conduct of this research study. Other staff members making important contributions to this work are: Nancy Blythe, Ann Bradshaw, Dante del Pilar, Nicole Donovan, Fran Fleck, Joia Hicks, Amy Hoffman, Dick Jacke, Kristine Maddux, Evan McKay, Susan McKeeth, Sarah Moore, Kathryn Nord, Patty Pride, Georgene Ranney, Tiffany Silver-Brace, Camille Taylor, Jodi Thiel, Loni Tipton, and Margaret Trzyna. Lastly, we want to acknowledge the time and generosity of all of the women who participated in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C.I., Beaber, E.F., Tang, MT.C. et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat 137, 579–587 (2013). https://doi.org/10.1007/s10549-012-2365-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2365-1