Abstract
There is a need for new biomarkers to more correctly identify node-negative breast cancer patients with a good or bad prognosis. Myristoylated alanine-rich C kinase substrate like-1 (MARCKSL1) is a membrane-bound protein that is associated with cell spreading, integrin activation and exocytosis. Three hundred and five operable T1,2N0M0 lymph node-negative breast cancer patients (median follow-up time 121 months, range 10–178 months) were evaluated for MARCKSL1 expression by immunohistochemistry and quantitative real-time PCR. The results were compared with classical prognosticators (age, tumor diameter, grade, estrogen receptor, and proliferation), using single (Kaplan–Meier) and multivariate survival analysis (Cox model). Forty-seven patients (15 %) developed distant metastases. With single and multivariate analysis of all features, MARCKSL1 protein expression was the strongest prognosticator (P < 0.001, HR = 5.1, 95 % CI = 2.7–9.8). Patients with high MARCKSL1 expression (n = 23) showed a 44 % survival versus 88 % in patients with low expression at 15-year follow-up. mRNA expression of MARCKSL1 in formalin fixed paraffin-embedded tissue was also prognostic (P = 0.002, HR = 3.6, 95 % CI = 1.5–8.3). However, the prognostic effect of high and low was opposite from the protein expression, i.e., low expression (relative expression ≤ 0.0264, n = 76) showed a 79 % survival versus 92 % in those with high expression of MARCKSL1 mRNA. Multivariate analysis of all features with distant metastases free survival as the end-point showed that the combination of MARCKSL1 protein and phosphohistone H3 (PPH3) has the strongest independent prognostic value. Patients with high expression (≥13) of PPH3 and high MARCKSL1 protein had 45 % survival versus 78 % survival for patients with low MARCKSL1 protein expression and high expression (≥13) of PPH3. In conclusion, MARCKSL1 has strong prognostic value in lymph node-negative breast cancer patients, especially in those with high proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a leading cause of female cancer mortality in the western world, second only to lung cancer. Improvement of survival with adjuvant systemic chemotherapy (AST) is less pronounced in lymph node-negative patients than in lymph node-positive patients: the typical 15-year survival improvement is 35 % relative and 10 % absolute [1]. Discomfort, costs, and the sometimes serious side effects of AST must be balanced against the relatively good prognosis and moderately favorable prognostic treatment effect in this subgroup of patients. Accurate and reliable prognostic markers would be valuable in decision-making regarding AST or not.
Previous studies have shown that proliferation assessed by either mitotic activity index (MAI) or phosphohistone H3 (PPH3) has strong prognostic value. Patients with a high proliferation index have a 3.5–5 times higher risk of developing and dying from distant metastases than those with a low proliferation [2]. However, in spite of their prognostic superiority, neither MAI nor PPH3 provide an optimal accuracy and only 30–40 % of patients with high proliferation die from their disease. It is therefore important to evaluate if other biologically features have independent or additional prognostic value.
Invasion of cells through the extracellular matrix (ECM) is essential for malignant development. This multistep process involves migration by two alternating modes of action: cellular deformation allowing movement through the ECM, or degradation of the ECM using factors such as matrix metalloproteinases [3, 4]. Another protein which has been implicated in cell motility, cell adhesion, phagocytosis, membrane traffic, and mitogenesis is the myristoylated alanine-rich C kinase substrate (MARCKS) [5] and MARCKS-like 1 (MARCKSL1). MARCKS and MARCKSL1 are both members of the MARCKS family of myristoylated protein kinase C (PKC) substrates that bind calmodulin after phosphorylation by PKC. All MARCKS family proteins bind to the plasma membrane through their N-terminal myristoylation tag and the effector domain, while they differ in sub-cellular localization and membrane-binding affinity (reviewed by [6]). MARCKSL1 is mainly expressed in the brain and reproductive tissue along with induced level of expression in macrophages upon bacterial lipopolysaccharide stimulation [7–9]. Although both MARCKS and MARCKSL1 are expressed in the brain, they show different regional localization and expression patterns during development, suggesting that they are uniquely regulated and potentially support distinctive functions [10, 11]. Epithelial cells endogenously express MARCKSL1, while fibroblasts have been reported to lack mRNA encoding for MARCKSL1 [9, 12]. MARCKS and, less pronounced, MARCKSL1 are also found to be involved in both bundling and cross-linking actin [13]. Furthermore, MARCKS and MARCKSL1 are critical for endothelial cell barrier enhancement upon sphingosine 1-phosphate stimulation [14]. In in vitro co-culture experiments, between breast cancer and bone cells, MARCKSL1 was the most significantly altered gene in response to estradiol in estrogen receptor alpha (ERα)-positive breast cancer cells [15].
In this study, we have evaluated whether MARCKSL1 mRNA- and/or protein expression has prognostic value in lymph node-negative breast carcinomas, either alone or in combination with steroid hormone receptors, proliferation, and other classical clinicopathological features.
Materials and methods
Patients and pathology
Prior to commencement the study was approved by the Regional Ethics Committee. The guidelines for reporting tumor marker studies were followed [16]. All 324 patients were diagnosed with invasive, operable (T1,2N0M0) breast cancer at the Stavanger University Hospital, between January 1, 1993 and December 31, 1998. In 103 patients, both fresh frozen tissue and FFPE tissue were available while for 221 patients only FFPE tissue was available. In 17 cases there was not enough tumor material, one sample having poor fixation and one having too much inflammation, leaving 305 patients for analysis (Fig. 1). There were no significant differences between the original 324 and final 305 cases in any of the features analyzed. The patients were all treated according to the national guidelines of the Norwegian Breast Cancer Group. The tumor size was measured in the fresh specimens following excision and cut in slices of 0.5-cm thick. The axillary fat was macroscopically examined and all detectable lymph nodes were prepared for histology. The median number of identified lymph nodes was 12 (range, 1–27). All tissues were fixed in buffered 4 % formaldehyde and embedded in paraffin, furthermore in 103 patients representative samples of the primary tumors were snap-frozen in liquid nitrogen and stored at −80 °C. Four micrometer histological sections were made and stained with hematoxylin–erythrosine–safran (HES). Histological type and grade were assessed by two pathologists (EG and JB) according to the World Health Organization criteria [17]. The MAI was assessed as described elsewhere [18].
Immunohistochemistry
ER and progesterone receptor (PR), PPH3, Ki67, cytokeratin 5/6 (CK5/6), HER2, and MARCKSL1 expressions were determined by immunohistochemistry (IHC) in whole sections. Antigen retrieval and IHC techniques were based on DAKO technology as described previously [19]. In brief, paraffin-embedded sections with 4 µm thickness, adjacent to the HES sections used for diagnosis, were mounted onto silanized slides (#S3002, DAKO, Glostrup, Denmark). Antigen was retrieved with a highly stabilized retrieval system (ImmunoPrep; Instrumec, Oslo, Norway) using 10 mM Tris/1 mM EDTA (pH 9.0) as the retrieval buffer. Sections were heated for 3 min at 110 °C followed by 10 min at 95 °C then cooled to 20 °C. ER (clone SP1, Neomarkers/LabVision, Fremont, CA, USA) was used at a dilution 1/400. PR (clone SP2, Neomarkers/LabVision) was used at a dilution of 1/1,000. Rabbit polyclonal anti-PPH3 (ser 10) (Upstate #06-570; Lake Placid, NY) was used at a dilution of 1:1,500. Ki67 (clone MIB-1, DAKO, Glostrup, Denmark) was used at dilution 1:100. CK 5/6 (Clone D5/16 B4, Dako, Glostrup, Denmark) was used at a dilution of 1/100. Mouse monoclonal MARCKSL1 (Clone K53, sc-130471, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:300. All antibodies were incubated for 30 min at 22 °C. The EnVision™ FLEX detection system (Dako, K8000) was used for visualization. Sections were incubated for 5 min with peroxydase-blocking reagent (SM801), 30 min with the primary antibody, 20 min with the EnVision™ FLEX/HRP Detection Reagent (SM802), 10 min with EnVision™ FLEX DAB+ Chromogen (DM827)/EnVision™ FLEX Substrate Buffer (SM803) mix and 5 min with EnVision™ FLEX Hematoxylin (K8008). The slides were dehydrated and mounted. All immunohistochemical stainings were performed using a Dako Autostainer Link 48 instrument and EnVision™ FLEX Wash Buffer (DM831). For HER2 assessments, DAKO HercepTest™ was used according to the procedures of the manufacturers.
Quantification of PPH3, CK5/6, ER, PR, Ki67, HER2, and MARCKSL1
The PPH3 index was assessed as described elsewhere [20]. PPH3 expression was evaluated using the fully automated VIS analysis system (Visiopharm, Hørsholm, Denmark), using the same image processing principles described previously [19].
For measuring percentage of Ki67 positive cells, the semi-automatic interactive computerized QPRODIT system (Leica, Cambridge) was used as described before [21]. For each measurement 200 fields of vision were selected, the Ki67 percentage was defined as [(Ki67 positive)/(Ki67 positive + Ki67 negative)] × 100.
The percentage of CK5/6 positive tumor cells in each tumor was scored using a continuous scale of 0–100 %. In the final analysis, all tumors with any CK5/6 staining in tumor cells were grouped as being positive as described before [22]. ER and PR were scored as positive when nuclear staining was present in >10 %, borderline 1–10 % and scored negative when <1 % of the tumor cells had nuclear staining. HER2 was scored according to the DAKO HercepTest scoring protocol. All 2+ and 3+ cases were regarded as positive. All sections were independently scored by two of the authors.
For MARCKSL1, the slides were scored using the following criteria: overall diffuse cytoplasmic staining, membrane staining, and granular staining in 10 high power fields (1.59 mm2), usually the invasive front of the tumor. For each of the criteria scoring from 0 to 3+ (0 = lowest score, 3+ = highest score) was given by assessing both intensity and number of positive tumor cells. For the membrane staining, the Dako HER2 scoring guideline was used. A total MARCKSL1 score was calculated by adding all the scores from the different criteria, resulting in a minimum score of 0 and a maximum score of 9. The slides were scored blinded and separately by two of the authors (DJ and IS).
RNA isolation and qPCR for FFPE tissue
All sections were evaluated by an experienced breast pathologist (EG) in order to assess the percentage of tumor cells in each tissue. All tissues contained at least 50 % tumor cells in the area selected for RNA isolation. Five sections of 10 μm were used for RNA isolation, in some cases a selected area was isolated by macrodissection from the slides. Total RNA was isolated using miRNeasy for FFPE Kit (Qiagen, Valencia, CA) according to the protocol provided by the manufacturer. For quality control all samples were analyzed on a Nanodrop Instrument (Thermo Fisher Scientific, Waltham, USA). cDNA samples were made out of 500 ng total RNA using the High Capacity cDNA RT Kit (Applied Biosystems, Carlsbad, California), following the manufacturers protocol. Two microliter of diluted cDNA (1:5) was used with the QuantiTect Syber Green PCR Kit (Qiagen), to a final reaction volume of 20 μl. Real-time PCR quantification was performed with a Light Cycler 480 (Roche, Zurich, Switzerland), using 5-μM MARCKSL1 specific primers (forward primer: GAAGGCCAACGGCCAGGAGA, reverse primer: CAGGGGGCGACTCCCCTTCA) and 5 μM of GAPDH specific primers (forward primer: TCTTTTGCGTCGCCAGCCGA, reverse primer: CGACCAAATCCGTTGACTCCGACC) (Eurofins MWG GmbH, Ebersberg, Germany).
To determine the relative expression level of each target gene, the comparative Ct method was used. The Ct value of the target gene was normalized by an endogenous reference (ΔCt = Ct(MARCKSL1)−Ct(GAPDH)). The relative expression of each target gene was calculated through the equation 2−ΔCt.
RNA isolation and qPCR from fresh frozen material
For all samples a new cryosection was cut and HE-stained, these sections were evaluated by an experienced breast pathologist (EG) in order to assess the percentage of tumor cells in the tissue. All tissues contained at least 50 % tumor cells in the selected area for RNA isolation. Five cryosections of 10 μm were used for RNA isolation, for some cases a selected area was isolated by macrodissection from the slides. Total cellular RNA was extracted from the frozen breast cancer tissues with mirVana miRNA Isolation Kit (Applied Biosystems/Ambion, Austin, USA). RNA samples were evaluated for integrity of 18S and 28S rRNA, and concentration by Bioanalyzer (Agilent Technologies, Santa Clara CA, USA) and Nanodrop Instrument (Thermo Fisher). Reverse transcription was completed with High Capacity cDNA Reverse Transcription Reagents Kit (Applied Biosystems). Two microgram of total RNA was transcribed into cDNA in a 20 μl reaction using random hexamers under the thermal condition recommended by the protocol. The cDNA was diluted 1:20 for the qPCR with QuantiTech SybrGreen PCR Kit (Qiagen), using 5-μM MARCKSL1 specific primers (forward primer: TTCTTTTCCAAGTAGGTTTTGTTTACC, reverse primer: CACTCAAGGTTTGGGAGTATAAGCA) and 5-μM GAPDH specific primers as the endogenous reference (forward primer: TGCACCACCAACTGCTTAGC, reverse primer: GGCATGGACTGTGGTCATGAG) (Eurofins MWG GmbH, Ebersberg, Germany). The Light Cycler 480 (Roche, Zurich, Switzerland) was used for the analysis.
The relative expression of MARCKSL1 gene was calculated via the equation 2−ΔCt as previously described.
qPCR of microRNAs
Six FFPE samples with low expression of mRNA and high protein expression of MARCKSL1 and six FFPE samples with both high mRNA and protein expression for MARCKSL1 were analyzed for relative expression of mir-23a, mir-23b, and mir-340. These three microRNAs are linked to posttranscriptional regulation of MARCSKL1 mRNA, according to the searching database “starBase” [23]. A 20 ng quantity of total RNA was reverse transcribed in a 20 μl volume using the Universal cDNA Synthesis Kit (Exiqon A/S, Vedbaek, Denmark) according to the manufacturer’s recommendations. An 8 μl volume of 80× dilution of cDNA was used in each of the real-time PCRs with SYBR® green master mix and microRNA LNA™ PCR primer sets (both from Exiqon A/S), following the manufacturers instructions. The average of Ct values of hsa-mir-24 and hsa-mir-26b were used as the endogenous reference and relative expression of target genes was calculated via the equation 2−ΔCt.
Survival endpoints
For survival analysis, the main end points were distant metastases free survival (DMFS). To determine the probability that patients would remain free from distant metastasis, we defined recurrence as any recurrence at a distant site. Patients were censored, from the date of the last follow-up visit, for death from causes other than breast cancer local or regional recurrences, and the development of a second primary cancer, including contralateral breast cancer. If a patient’s status during follow-up indicated a confirmed metastasis without a recurrence date, the last follow-up visit date was used. Age, time to first recurrence and survival time were calculated relative to the primary diagnosis date.
Statistical analyses
SPSS (SPSS, Chicago, IL, USA) version 18.0 was used. The optimal expression threshold for MARCKSL1 mRNA and protein expression for detection of DMFS was determined by receiver operating curve (ROC) analysis (MedCalc statistical software v. 9.3.7, MedCalc, Mariakerke, Belgium). The cut-off with the best possible sensitivity and specificity was chosen. Kaplan–Meier survival curves were constructed and differences between groups were tested by the log rank test. The relative importance of potential prognostic variables was tested using Cox-proportional hazard analysis (forward, Wald) and expressed as a hazards ratio (HR) with 95 % confidence intervals.
Results
Median age for the 305 patients was 58 (range 30–84 years) with a median follow-up of 121 months (range 10–178 months). In total 47/305 (15 %) developed distant metastases and 33 (11 %) died of breast cancer related disease. Table 1 shows the survival and hazard ratios for the tumor characteristics for DMFS. Strong prognostic factors were MAI, Ki67, PPH3, MARCKSL1 protein, and MARCKSL1 mRNA. Tumor diameter, Nottingham grade and HER2 were less strong. Among the biomarkers for proliferation PPH3 was the strongest (HR = 3.8, P < 0.001, 95 % confidence interval (CI) = 2.0–7.2). Patients with PPH3 < 13 had 92 versus 73 % survival for patients with PPH3 ≥ 13 (Fig. 2A).
For MARCKSL1 mRNA expression analysis, 51 cases were excluded because of a too low concentration of total RNA. This did not cause any significant differences in any of the features analyzed. Since the quality of mRNA in fresh frozen material and FFPE is very different, the ROC analyses were preformed separately for the two types of material. MARCKSL1-threshold correlated to DMFS was 0.036 for fresh frozen material and 0.026 for FFPE material. Using these thresholds, the patients were divided into groups with low and high MARCKSL1 mRNA expression. With univariate DMFS survival analysis, high (n = 33) and low (n = 55) MARCKSL1 mRNA expression in fresh frozen material had 88 versus 66 % survival, respectively. For FFPE material, the survival was 92 and 79 % survival for patients with high (n = 108) and low expression (n = 76) (Fig. 2C and D).
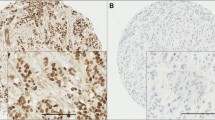
In all 305 patients, MARCKSL1 protein was identified in either the membrane, or granular and diffuse cytoplasmic staining in cancer cells (Fig. 3). A cancer cell did not have both strong membrane and granular staining. Normal epithelial cells showed some (total score of 4–5) staining (mostly diffuse cytoplasmic) as well. With ROC analysis, a MARCKSL1-total score threshold of 7 or higher correlated strongest to DMFS. Using this threshold, the patients were divided into low and high MARCKSL1 expressions (n = 283, 93 % and n = 23, 7 %). Table 1 shows that immunohistochemically identified protein expression of MARCKSL1 is the single most prognostic factor with a HR = 5.1 (P < 0.001, 95 % CI = 2.7–9.8). DMFS survival analysis of the different staining patterns of MARCKSL1 showed that membrane and granules both had significant results. Patients with high MARCKSL1 protein expression were also associated with ER positivity (16 of 23 patients) and were often under 55 years old (16 of 23 patients). mRNA expression of MARCKSL1 showed the opposite results of protein expression, with low and high protein expression of MARCKSL1 being 88 versus 44 % for DMFS, respectively.
Example of MARCKSL1 staining (brown staining). A The arrow is pointing at strong membrane staining (score: membrane 3+, granular 0 and diffuse 1+), B the arrow is pointing at strong granular staining (score: membrane 0, granular 3+, and diffuse 2+), C strong diffuse staining (score: membrane 0, granular 1+, and diffuse 3+), D moderate diffuse staining (score: membrane 0, granular 0, and diffuse 2+), E weak/negative diffuse staining (score: membrane 0, granular 1+ and diffuse 0), and F normal ducts and lobule with weak staining (score: membrane 0, granular 3+ and diffuse 1+)
In the multivariate analyses, we included all the variables that showed a significant correlation with DMFS in univariate analysis. MARCKSL1 mRNA expression was excluded due to insufficient cases. MARCKSL1 protein expression and PPH3 were the strongest prognostic factors for DMFS (n = 300, 98 %). MARCKSL1 protein expression had additional prognostic information in the group of patients with PPH3 ≥13 (Fig. 2E), 78 versus 45 % (P = 0.001) for low expression of MARCKSL1 protein.
In a small group of patients without any adjuvant treatment and low proliferation (MAI < 10), Kaplan–Meier analysis with MARCKSL1 protein expression results in DMFS of 92 versus 25 % survival.
None of the microRNAs analyzed (mir-23a, mir-23b, and mir-340) showed a significant correlation to MARCKSL1 mRNA or protein expression in the two groups of tumors, one with both high protein and mRNA MARCKSL1 expression and one with low protein MARCKSL1 and high mRNA expression.
Discussion
In this study, we tested whether MARCKSL1 mRNA and/or protein expression could be useful biomarkers for prediction of DMFS in patients with operable lymph node-negative breast cancer. Combined high MARCKSL1 protein and high PPH3 had the strongest independent prognostic value, while high MARCKSL1 protein expression alone was the strongest single variable associated with a very poor prognosis.
The MARCKS proteins, MARCKS and MARCKSL1 are widely distributed membrane-binding proteins that are major PKC-substrates. After phosphorylation, MARCKSL1 is relocated to the intracellular membranes of late endosomes and lysosomes. MARCKSL1 binds to all membranes via its myristoyl moiety, but for its localization at the plasma membrane the effector domain is also required [24]. Analysis of the protein expression by IHC in our study shows MARCKSL1 expression as a clear membrane staining, diffuse cytoplasmic staining and more punctuated (granular) cytoplasmic staining. The granular staining we observed, fits well with the lysosomal translocation of MARCKSL1 after phosphorylation observed by van de Bout et al. [25]. In our study, both the granular and the membrane staining are equally prognostic, and therefore no conclusions can be made regarding which localization of MARCKSL1 is the most important for breast cancer metastasis.
Generally, high protein expression of MARCKSL1 was associated with higher risk for metastasis, especially in the subgroup of ERα-positive tumors and in the, already high risk, subgroup of highly proliferating tumors. Sixteen of the twenty-three cases with high protein expression for MARCKSL1 occurred in the group of highly proliferating ERα-positive tumors and 11 of these developed distant metastasis. Wang et al. [15] also showed that MARCKSL1 was one of the genes with the highest expression in ER-positive tumors, while investigating the effects of 17-β estradiol in a co-culture model system with breast cancer and bone cells. They hypothesized that MARCKSL1 is also a potential marker for patients at high risk of bone metastasis. Data from other studies also support a role for MARCKSL1 in tumor metastasis [5, 26]. Experiments on macrophage cells showed that MARCKSL1 in collaboration with dynamitin, a protein involved in microtubule function, is essential for cell spreading [5]. On the other hand, Finlayson et al. [27] suggested that knockdown of MARCKSL1, in both EpRas mammary epithelial cells and PC3 prostate cancer cells, promoted in vitro cell migration that was blocked by calmodulin inhibitor, trifluoperazine. During wound healing experiments, they observed that reduced MARCKSL1 supported collective cell migration and promotes adherens junction formation.
mRNA expression patterns analyzed from both fresh frozen tissue and FFPE tissue showed that low MARCKSL1 mRNA expression was associated with a high risk for developing distant metastasis. Interestingly, the opposite results were found for MARCKSL1 protein expression where high expression is correlated with a worse prognosis. According to the central dogma of molecular biology, one would assume that mRNA abundance can be directly correlated with protein levels. However, this study showed no correlation between MARCKSL1 mRNA and protein expression. Other groups have showed that the correlation between mRNA expression and protein expression can vary from as little as 17 to 33 % [28, 29]. A reason for the lack of correlation could be the fact that the mRNA is isolated from a selection of cells that contain >50 % tumor cells but still contains a population of non-tumor cells this in contrast to the protein expression levels that were assessed in the tumor cells only. Another reason for the lack of correlation between the mRNA and protein expression levels might be the heterogeneity of the tumors, both protein and mRNA expression levels are different in different areas of a tumor. However, more important biologically, the causes might be the regulation of mRNA expression by a negative feedback loop or by posttranscriptional regulation through microRNA. MicroRNAs are short non-coding RNAs that regulate/inhibit the mRNA translation by binding to the mRNA (reviewed in [30]). In our study, the expression of mir-23a, mir-23b, and mir-340 could not explain the difference in protein expression in this small group of patients with high MARCKSL1 mRNA expression.
In conclusion, our results show that both high proliferation and high MARCKSL1 protein expression are correlated with an increased risk for metastasis and a worse prognosis in lymph node-negative breast cancer patients. As this is the first study to report about the prognostic value of MARCKSL1 in breast cancer we used the optimal cut-off value from our ROC-analysis, to stratify our patients in good or bad prognosis. This cut-off could very much be related to our current patient cohort and should therefore be validated in another cohort of independent breast cancer patients. The biological role of MARCKSL1 needs further investigation as phosphorylation might play an important role in both localization and action of MARCKSL1.
References
Early Breast cancer Trialists’ collaborative group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Baak JP, van Diest PJ, Voorhorst FJ, van der Wall E, Beex LV, Vermorken JB, Janssen EA (2005) Prospective multicenter validation of the independent prognostic value of the mitotic activity index in lymph node-negative breast cancer patients younger than 55 years. J Clin Oncol 23(25):5993–6001. doi:10.1200/JCO.2005.05.511
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P (2003) Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160(2):267–277. doi:10.1083/jcb.200209006
Pandey PR, Saidou J, Watabe K (2010) Role of myoepithelial cells in breast tumor progression. Front Biosci 15:226–236
Yue L, Lu S, Garces J, Jin T, Li J (2000) Protein kinase C-regulated dynamitin-macrophage-enriched myristoylated alanine-rice C kinase substrate interaction is involved in macrophage cell spreading. J Biol Chem 275(31):23948–23956. doi:10.1074/jbc.M001845200
Arbuzova A, Schmitz AA, Vergeres G (2002) Cross-talk unfolded: mARCKS proteins. Biochem J 362(Pt 1):1–12
Blackshear PJ, Verghese GM, Johnson JD, Haupt DM, Stumpo DJ (1992) Characteristics of the F52 protein, a MARCKS homologue. J Biol Chem 267(19):13540–13546
Li J, Aderem A (1992) MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell 70(5):791–801. doi:10.1016/0092-8674(92)90312-Z
Lobach DF, Rochelle JM, Watson ML, Seldin MF, Blackshear PJ (1993) Nucleotide sequence, expression, and chromosomal mapping of Mrp and mapping of five related sequences. Genomics 17(1):194–204. doi:10.1006/geno.1993.1301
McNamara RK, Stumpo DJ, Morel LM, Lewis MH, Wakeland EK, Blackshear PJ, Lenox RH (1998) Effect of reduced myristoylated alanine-rich C kinase substrate expression on hippocampal mossy fiber development and spatial learning in mutant mice: transgenic rescue and interactions with gene background. Proc Natl Acad Sci USA 95(24):14517–14522
Stumpo DJ, Eddy RL Jr, Haley LL, Sait S, Shows TB, Lai WS, Young WS 3rd, Speer MC, Dehejia A, Polymeropoulos M, Blackshear PJ (1998) Promoter sequence, expression, and fine chromosomal mapping of the human gene (MLP) encoding the MARCKS-like protein: identification of neighboring and linked polymorphic loci for MLP and MACS and use in the evaluation of human neural tube defects. Genomics 49(2):253–264. doi:10.1006/geno.1998.5247
Myat MM, Chang S, Rodriguez-Boulan E, Aderem A (1998) Identification of the basolateral targeting determinant of a peripheral membrane protein, MacMARCKS, in polarized cells. Curr Biol 8(12):677–683
Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A (1992) MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356(6370):618–622. doi:10.1038/356618a0
Guo Y, Singleton PA, Rowshan A, Gucek M, Cole RN, Graham DR, Van Eyk JE, Garcia JG (2007) Quantitative proteomics analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol Cell Proteomics 6(4):689–696. doi:10.1074/mcp.M600398-MCP200
Wang J, Jarrett J, Huang CC, Satcher RL Jr, Levenson AS (2007) Identification of estrogen-responsive genes involved in breast cancer metastases to the bone. Clin Exp Metastasis 24(6):411–422. doi:10.1007/s10585-007-9078-6
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23(36):9067–9072. doi:10.1200/JCO.2004.01.0454
World Health Organization (1981) Histologic typing of breast tumors, 2nd edn. World Health Organization, Geneva
Baak JP, van Diest PJ, Ariens AT, van Beek MW, Bellot SM, Fijnheer J, van Gorp LH, Kwee WS, Los J, Peterse HC et al (1989) The multicenter morphometric mammary carcinoma project (MMMCP). A nationwide prospective study on reproducibility and prognostic power of routine quantitative assessments in The Netherlands. Pathol Res Pract 185(5):664–670
Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP (2009) Validating the prognostic value of proliferation measured by phosphohistone H3 (PPH3) in invasive lymph node-negative breast cancer patients less than 71 years of age. Breast Cancer Res Treat 114(1):39–45. doi:10.1007/s10549-008-9980-x
Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP (2007) Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol 20(12):1307–1315. doi:10.1038/modpathol.3800972
Bol MG, Baak JP, Rep S, Marx WL, Kruse AJ, Bos SD, Kisman O, Voorhorst FJ (2002) Prognostic value of proliferative activity and nuclear morphometry for progression in TaT1 urothelial cell carcinomas of the urinary bladder. Urology 60(6):1124–1130
Skaland I, Janssen EA, Gudlaugsson E, Hui Ru, Guo L, Baak JP (2009) The prognostic value of the proliferation marker phosphohistone H3 (PPH3) in luminal, basal-like and triple negative phenotype invasive lymph node-negative breast cancer. Cell Oncol 31(4):261–271. doi:10.3233/CLO-2009-0464
Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH (2011) starBase: a database for exploring microRNA–mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res 39(Database issue):D202–D209. doi:10.1093/nar/gkq1056
van den Bout I, Truong HH, Huveneers S, Kuikman I, Danen EH, Sonnenberg A (2007) The regulation of MacMARCKS expression by integrin beta3. Exp Cell Res 313(6):1260–1269. doi:10.1016/j.yexcr.2007.01.005
van den Bout I, van Rheenen J, van Angelen AA, de Rooij J, Wilhelmsen K, Jalink K, Divecha N, Sonnenberg A (2008) Investigation into the mechanism regulating MRP localization. Exp Cell Res 314(2):330–341. doi:10.1016/j.yexcr.2007.08.019
Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A, Miwa M, Saya H, Yongvanit P (2010) Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci 101(3):658–665. doi:10.1111/j.1349-7006.2009.01427.x
Finlayson AE, Freeman KW (2009) A cell motility screen reveals role for MARCKS-related protein in adherens junction formation and tumorigenesis. PLoS One 4(11):e7833. doi:10.1371/journal.pone.0007833
Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 1(4):304–313
Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M, Nilsson P (2009) Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 10:365. doi:10.1186/1471-2164-10-365
Mattick JS, Makunin IV (2006) Non-coding RNA. Hum Mol Genet 15(spec no 1):R17–R29. doi:10.1093/hmg/ddl046
Acknowledgments
Kristin Jonsdottir and Benny Björkblom have grants from Helse Vest, Norway. Eleanor T. Coffey is supported by grant #218125 from the Academy of Finland. This study has received financial support from the Folke Hermansen Foundation in 2007 and grants from the Stavanger University Hospital research department in 2009 and 2010.
Conflicts of interest
None of the authors have any financial or other relationships with entities that have investment, licensing, or other commercial interests in the subject matter under consideration in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jonsdottir, K., Zhang, H., Jhagroe, D. et al. The prognostic value of MARCKS-like 1 in lymph node-negative breast cancer. Breast Cancer Res Treat 135, 381–390 (2012). https://doi.org/10.1007/s10549-012-2155-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2155-9