Abstract
To assess the prognostic value of presurgical CA15.3 in a large cohort of patients with early breast cancer. A total of 7.942 consecutive patients with breast cancer operated at the European Institute of Oncology between 1998 and 2005 and with presurgical values of CA 15.3 available were included. We explored patterns of recurrence by baseline CA 15.3 values. Mean CA15.3 was 17.0 U/ml. CA15.3 was associated with age, tumor size, nodal involvement, Ki-67 labeling index, grade, HER2 expression, molecular subtype, and perivascular invasion. CA15.3 was independently associated with distant metastases [HR > 20 U/ml vs. ≤ 20 U/ml: 1.34 (95% CI 1.15–1.56)] and death [HR > 20 U/ml vs. ≤ 20 U/ml: 1.30 (95% CI 1.11–1.53)]. When considering CA15.3 as continuous variable, we observed a constant risk of metastasis and death from the lowest values to about 15–20 U/ml, and then a significantly increasing risk with increasing values of CA15.3. Finally, CA15.3 provided significant additional information to the common prognostic factors to predict the occurrence of metastases (C-index P value 0.04). In patients with operable breast cancer, presurgical CA15.3 value is an independent prognostic factor for metastases and deaths. CA15.3 provides additional information to the common prognostic factors and should be considered in the adjuvant therapeutic algorithm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum tumor markers are routinely used in the management of patients with different cancers [1]. In breast cancer patients carbohydrate antigen 15.3 (CA15.3) is considered the serum marker of choice. It is a transmembrane glycoprotein encoded by the MUC1 gene, defined by reactivity with two monoclonal antibodies, DF3 and 115-D8, in a sandwich immunoassay [2]. MUC1 gene is involved in cell adhesion, reducing cell to cell aggregation, and cell adherence to extracellular matrix [3], induces an immunosuppressive action [4–6] and therefore facilitates metastasis formation [7, 8]. It is heterogeneously expressed on the apical surface of different normal epithelial cell types, but it is aberrantly overexpressed in 90% of breast cancer [9].
CA 15-3 is elevated in patients with distant metastasis, but rarely in patients with early breast cancer. Owing to its low sensitivity in early stages, the use of this marker as a screening or diagnostic tool is not recommended. In 2007, the last update of the American Society of Clinical Oncology (ASCO) regarding the use of tumor markers in breast cancer, recommends that CA15.3 should be used to monitor patients with advanced breast cancer, especially in case of a disease difficult to evaluate with standard criteria [10]. On the other hand, although several studies [11–20] supported its clinical prognostic value in early stage breast cancer, strong evidence is still lacking. Therefore, the ASCO panel stated that the data available were insufficient to recommend the use of CA15.3 for screening, diagnosis, staging, and monitoring for recurrence after primary breast cancer therapy [10].
The aim of this study was to clarify the prognostic value of preoperative CA15.3 determination in a large number of patients with operable breast cancer undergoing curative surgery, for whom a long follow-up was available. To this end we reviewed the relationship between the level of the marker with (i) clinico-pathological parameter, and (ii) clinical outcome in a series of 7,942 patients.
We explored patterns of recurrence also according to preoperative CA15.3 values in immunohistochemically defined tumor subtypes. In fact recommendations for selection of adjuvant systemic treatments in patient subpopulations were recently proposed based on the recognition of biological subtypes with specific responses to systemic and local therapies [21]. Five groups (Luminal A, Luminal B HER2 negative, Luminal B HER2 positive, HER2 positive (non-luminal), and triple negative) were identified based on expression of estrogen (ER) and progesterone receptor (PgR), overexpression and/or amplification of the HER2 oncogene, and Ki-67 labeling index [22]. The recognition of specific prognostic factors for a specific immunohistochemically defined tumor subtype might be relevant for the development of tailored adjuvant treatment options.
Patients and methods
We collected information on all consecutive breast cancer patients candidate for surgical treatment at the European Institute of Oncology between July 1998 and December 2005. All cases were prospectively entered into the IEO breast cancer database and discussed at the weekly multidisciplinary meeting, which is attended by breast surgery, medical oncology, radiotherapy, and pathology specialists and which results in a proposal for postoperative adjuvant treatments. We excluded male patients, patients with synchronous distant metastases, bilateral or recurrent tumor, previous cancer, and those receiving primary medical treatment. Overall, 8.827 women were selected. Among them, 7.942 (90%) had also the pre-surgical CA15.3 determined, and represented this study population.
Pathological assessment included evaluation of the primary tumor size, histological type, and of lymph nodes status including a sentinel node biopsy, when applicable. Tumor grade, peritumoral vascular invasion (PVI), ER, PgR, and Ki-67 labeling index were determined by immunohistochemistry (IHC) as previously described [23, 24] and recorded as the percentage of immunostained cells. HER2 was assayed by IHC and fluorescent in situ hybridization (FISH) using standard reagents and procedures [25] and was considered positive if FISH showed a ratio of HER2 to chromosome 17 of 2.0 or greater, or based only on IHC score of 3+ (in 0.5% of cases).
Blood samples were collected preoperatively from patients with a newly diagnosed breast cancer, attending clinical evaluation prior to surgical intervention. Blood specimens were allowed to clot before being centrifuged at 1100×g for 10 min and the serum values of CA15.3 were determined using the Abbott ASXYM system.
Each patient was followed for disease recurrence and survival status with a median follow-up of 75 months (range 1–147). The primary outcome was loco-regional disease-free survival (LRDFS), defined as the first breast cancer recurrence, secondary end points included distant disease-free survival (DDFS) and overall survival (OS).
The prognostic impact of CA15.3 was analyzed considering both the standard cut-point of 31 U/ml and the quartile values.
Patients underwent either a modified radical mastectomy [1.501 patients (18.9%)] or a partial mastectomy [quadrantectomies 6.441 patients (81.1%)].
Following surgery, all cases were discussed during the weekly multidisciplinary meeting attended by surgeons, medical oncologists, radiation oncologists, and pathologists. The proposal for adjuvant systemic treatment was made on the basis of biological features, staging, previous treatments, comorbidities, and patient’s preference: 4,297 (54.1%) patients received hormonotherapy alone, 2,275 (28.7%) hormonotherapy and chemotherapy, 976 (12.3%) chemotherapy alone, and 323 (4.1%) no adjuvant systemic treatment. Furthermore, 71 (0.9%) patients received target therapy (Trastuzumab) in combination with chemotherapy.
Statistical methods
Differences between means were tested using the Wilcoxon test, when comparing two groups, and the Kruskal–Wallis test, when comparing three or more groups. Recurrences within the same breast and/or the regional nodes were considered as loco-regional events. Distant metastases and deaths from breast cancer as first events were treated as distant recurrences. If patients had simultaneous loco-regional and distant events they were considered as having a distant recurrence. Crude cumulative incidences of loco-regional events were computed in a competing risk framework with distant events, contralateral tumors, and non-breast primary tumors being treated as competing events. For the crude cumulative incidences of distant events, loco-regional events, contralateral tumors, and non-breast primary tumors were treated as competing events. Cumulative incidences were compared across different subgroups by means of the Gray test [26]. Cumulative mortality was calculated as the cumulative incidence of deaths from any cause and the Log-rank test was used to assess survival differences between groups.
In order to investigate the shape of the relationship between CA15.3 and the hazard of events, Cox proportional hazard models were fitted, adjusted for age, pT, pN, HER2, ER, PgR, Ki-67, and IVP. CA15.3 was firstly modeled as a categorical variable. Then, restricted cubic spline models were used. Cubic splines are smoothly joined piecewise third-order polynomials [27]. Polynomials are fitted within intervals delimited by knots, and restrictions are placed on the resulting curve to insure a smooth appearance at the knot points. A four-knot analysis was performed. Results were presented in terms of Hazard ratio (HR), using the median value of CA15.3 as reference (HR = 1). Owing to the skewness of the distribution of CA15.3, the log-transformed data were used in the analyses and reported on the log scale in the figures.
Finally, the performance of the multivariable models was studied with respect to discrimination. Discrimination refers to the ability of the model to separate those who experience a distant metastasis from those who do not or to separate those who die and those who do not. It was quantified by a measure of concordance, the c-statistic. The c-statistic lies between 0.5 (no discrimination) and 1 (perfect discrimination). A Cox model including age, pT, pN, HER2, ER, PgR, Ki-67, and IVP was compared with a model including the same covariates and CA15.3. Differences in C statistics after the addition of CA15.3 were evaluated with the method described by Antolini et al. [28].
All analyses were carried out with the SAS software (SAS Institute, Cary, NC) and the R (http://cran.r-project.org/) software with the Harrell’s Design and Hmisc libraries. All the reported P values were two sided.
Results
CA15.3 and clinicopathological characteristics
Clinicopathological characteristics in association with pre-operative CA15.3 values are shown in Table 1. Overall, median and mean values of CA15.3 were 15.1 and 17.0 U/ml, respectively. CA15.3 was associated with age, tumor size, nodal involvement, Ki67, histological grade, HER2 expression, molecular subtype, and perivascular invasion. Other prognostic factors, such as histological type and ER/PgR receptor status, were not related to the preoperative concentration of CA15.3.
CA15.3 and outcome
After a median follow-up time of 75 months (range 15–147), 391 (4.9%) patients had a loco-regional recurrence, 758 (9.5%) had a distant metastasis and 702 (8.8%) patients died (5-year cumulative incidences were 3.9, 8.6, and 6.1%, respectively, Table 1). A detailed description of events is reported in Table 2. Among all deaths, 523 out of 702 (74.5%) were due to breast cancer, 126 (17.9%) to other causes, and 53 (7.6%) to unknown causes.
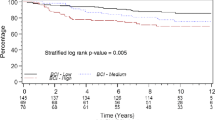
Univariate analysis is reported in Table 1. Age, histotype, tumor size, nodal involvement, ER and PgR status, Ki67, HER2, molecular subtype, tumor grade, perivascular invasion were significantly associated with all types of event. CA15.3 was a statistically significant prognostic factor for distant metastases and deaths (P < 0.01) using both the standard cut point of 31 U/ml and the quartiles of the distribution, but not for loco-regional events (P = 0.32 and P = 0.45 using the cut-point of 31 U/ml and the quartiles, respectively). Kaplan–Meier curves are shown in Fig. 1a, c–e. CA15.3 was a significant predictor of metastases and deaths also at multivariable analysis: HR > 20 U/ml vs. ≤ 20 U/ml: 1.34 (95% CI 1.15–1.56) for metastases and HR > 20 U/ml vs. ≤ 20 U/ml: 1.30 (95% CI 1.11–1.53) for deaths. We also performed the analysis including the standard cut-point of 31 U/ml and we obtained the following results: HR 20.1–31 U/ml vs. ≤ 20 U/ml: 1.26 (95% CI 1.06–1.49) and HR > 31 U/ml vs. ≤ 20 U/ml: 1.56 (95% CI 1.23–1.99) for metastases and HR 20.1–31 U/ml vs. ≤ 20 U/ml: 1.26 (95% CI 1.06–1.50) and HR > 31 U/ml vs. ≤ 20 U/ml: 1.44 (95% CI 1.11–1.86) for deaths.
Relationship between CA15.3 and survival. Cumulative incidence (%) of loco-regional events (a), distant metastases (c), and death (e) by CA15.3 quartiles. Continuous relationship between CA15.3 and the risk of loco-regional events (b), of distant metastases (d), and of death (e). Median value of CA15.3 set as the reference value (HR = 1). Dotted lines represent the 95% CIs
Twenty-four patients presented metastases within 3 months. Since in those cases metastatic disease could have already been present at diagnosis, we excluded them in a sensitivity analysis. Results did not change.
The continuous relationship between CA15.3 and risk of events was investigated through cubic splines multivariable models. With regards to loco-regional events (Fig. 1b), no significant increase or decrease in risk was observed with increasing values of CA15.3. In contrast, we observed an approximately constant risk of metastasis and death from the lowest values of CA15.3 up to about 15–20 U/ml, and then a significantly increasing risk with increasing values of CA15.3 (Fig. 1d, f, respectively). Accordingly with the above reported results, the prognostic value of CA15.3 was highly significant for both metastases and deaths (P < 0.01). The non-linear effect of CA15.3 was statistical significant for the metastases (P = 0.04) and borderline significant for deaths (P = 0.09).
Finally, we evaluated the ability of the Cox model including age, pT, pN, HER2, ER, PgR, Ki-67, and IVP as covariates to discriminate those who experienced a distant metastasis from those who did not and we obtained a C-index of 0.805 (Table 3). The same model including CA15.3 as an additional covariate lead to an increased C-index of 0.808. The difference between the two estimates was statistically significant (P = 0.04). The same figures for deaths were 0.793 and 0.796, with a P value of 0.11.
CA15.3 and outcome according to different molecular subtypes
The relationship between CA15.3 and the risk of death according to different molecular subtypes (i.e., Luminal A, Luminal B HER2-negative, Luminal B HER2-positive, HER2 positive non-luminal, and Triple negative) was evaluated (Fig. 2a–e). The relationship between CA15.3 and the risk of metastasis gave similar results and was not reported. Pre-operative CA15.3 levels were significantly associated with the risk of death in the subgroup of patients with Luminal B HER2-negative tumors and HER2-positive tumors non-luminal tumors (Fig. 2b, d). Interestingly, in patients with triple negative tumors we initially observed an increasing risk of death with increasing values of CA15.3. The increase rate lessened after the median value of CA15.3 (15.6 U/ml) and the adjusted HR stabilized on approximately 1. Triple negative patients with values of CA15.3 < 15.6 U/ml represented a subgroup with borderline statistically significant better prognosis compared to triple negative patients with higher values of CA15.3 [age, pT, pN, and IVP adjusted HR<15.6 vs. ≥15.6: 0.76 (95% CI 0.53–1.10)].
Discussion
The degree of preoperative CA 15.3 values is commonly not accounted for in the decision about adjuvant therapy. In fact, available data have several limitations.
The majority of papers suggest that increased CA15.3 is predictive of earlier relapse and reduced OS, but some other studies fail to demonstrate any statistically significant association between CA15.3 and prognosis [11–20]. The low sensitivity of this marker has precluded its use in the screening setting, while data in the presurgical samples are conflicting and do not reach a sufficient level of evidence to draw definite indications for the clinical practice. First of all, the small number of patients considered in the studies, usually <1000, which rises caution in the interpretation of the conclusions, and the length of the follow-up period which, when declared, is very variable among the different studies, ranging from around 3 to 10 years. Second, the kind of statistical analysis which, especially in the older papers, is only univariate, while in the more recent ones the multivariate approach is reported. For example, McLaughlin et al. [11] showed a lower probability of both relapse-free and OS in a small group of patients with high pre-operative of CA15.3 without performing a multivariate analysis; subsequently Canizares et al. [12] and Ebeling et al. [14], reported a significant correlation between the preoperative levels of CA15.3 and outcome in univariate but not in multivariate analysis. Only the more recent studies [15, 17–20] confirmed at the multivariate evaluation the results found at the univariate analysis. Finally different cut-points are used, ranging from 20.11 U/ml to 51 U/ml [18–20], although in the majority of the studies a threshold of 30 U/ml is considered; in spite of this, in all studies increased CA15.3 was significantly associated with worse prognosis in multivariate analysis, although in one study [18] CA15.3 was prognostic for relapse-free survival only in patients with node positive disease.
Tumor tissue markers are commonly used and well-accepted prognostic factors in breast cancer. Their determination provides information both to evaluate the prognosis and to decide the postsurgical treatment. However, different reports pointed out that they can be considerably affected by an inter-observer and inter-laboratory variability [23, 29]. Several studies [30–32] have highlighted discordant results among laboratories in the assessment of protein immunohistochemical expression due to inter-observer variability in the interpretation of the results and to differences in immunohistochemical procedures and cut-point adopted. These findings support the need for indicators that are accurate, standardized, reproducible, cheap, and easy to perform: CA15.3 fulfills these requirements, as it is a fast, non invasive, reproducible, objective, and quantitative test.
This study, based on prospectively defined and quality controlled database, provides the largest population of patients collected in a relatively short time and with long follow-up available to examine this issue. In this study we demonstrated that preoperative CA15.3 is an independent prognostic factor in patients with early breast cancer, and that an elevated preoperative level of CA15.3 resulted significantly associated with the development of distant metastases and death. We observed an approximately constant risk of metastasis and death from the lowest value of CA15.3 up to about 15–20 U/ml, and then a significantly increasing risk with increasing value of the marker. Moreover, when studying the risk of metastases, the addition of CA15.3 to the multivariable model including all the standard prognostic factors significantly increased the model’s discrimination ability (C-index P value: 0.04).
CA 15.3 is related to MUC1 expression, and it is shed into the bloodstream. Several studies have shown a different expression of mucins in breast cancer compared to the non-tumor breast tissue. Mucins act through the modulation of several signalling pathways and play a role in the progression of the disease, being involved in the regulation of the proliferative, invasive and metastatic activity of cancer cells [33]. Increased serum levels of CA15.3 may therefore mirror the presence of cells bearing a particularly aggressive phenotype. Moreover, it has already been suggested that a possible explanation for the association between CA15.3 and worse prognosis may be that the marker is elevated in patients with micrometastatic disease, which is not detected by standard radiological imaging.
This is the first study that evaluated the prognostic value of CA15.3 also within immunohistochemically defined subgroups of breast cancer. Perou et al. were the first to demonstrate that the phenotypic diversity of breast tumors was associated with corresponding gene expression diversity [34]. Using a subset of 456 genes from 65 tissue samples, the authors were then able to identify four different molecular subtypes of breast cancer: estrogen receptor (ER)-positive/luminal-like, basal-like, HER2-positive, and normal breast. Subsequent data expanded the classification to distinguish between luminal A and luminal B [35]. These five molecular classified subgroups correspond reasonably well to clinical-pathological characterization on the basis of estrogen receptor (ER) and HER2 status, as well as proliferation markers or histologic grade performed by means of immunohistochemistry (IHC) techniques [22, 36]. However, the tumor subtypes, recently identified, include heterogeneous groups of tumors and the detection, through new prognostic and predictive factors, of further subgroups amenable to targeted treatments represents a research priority.
We found a prognostic role for CA15.3 within the subgroups of patients with Luminal B HER2 negative disease and HER2 positive non-luminal disease.
The threshold indication for inclusion of chemotherapy for patients with Luminal B HER2 negative disease still represents an area of controversy. According to the results of this study baseline CA15.3 might be of value in the identification of higher risk of relapse where adjuvant chemotherapy might be introduced.
Moreover, a marginal positive trend for patients with triple-negative breast cancer and low Ki-67 was observed. Triple-negative breast cancer still represents an heterogeneous group of tumor with different outcome according to clinicopathological features [37]. The addition of a pre-surgery CA15.3 determination to the common used tissue marker might be helpful for the identification of subgroups of patients with triple-negative breast cancer and favorable outcome, where tailored adjuvant therapies should be studied.
In conclusion our analyses show explicitly that the presence of an abnormal CA15.3 pre-surgical value is associated with an increased risk of recurrence and death. Further studies using database analyses or prospective trials are required to confirm the prognostic value of pre-surgery CA15.3 determination in breast cancer. If confirmed, the presence of elevated CA 15.3 should be added to the list of features which must be taken into account while making a proper treatment choice.
References
Sturgeon C (2002) Practice guidelines for tumor marker use in the clinic. Clin Chem 48:1151–1159
Hilkens J, Buijs F, Hilgers J et al (1984) Monoclonal antibodies against human milk fat globule membranes detecting differentiation antigens of mammary gland and its tumor. Int J Cancer 34:197–206
Wesseling J, van der Valk SW, Hilkens J (1996) A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane associated mucin episialian/MUC1. Mol Biol Cell 7:565–577
Fung PYS, Longenecker BM (1991) Specific immunosuppressive activity of epiglycanin, a mucin like glycoprotein secreted by a murine mammary adenocarcinoma (TA3-HA). Cancer Res 51:1170–1176
Gimmi CD, Morrison BW, Mainprice BA et al (1996) Breast cancer associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat Med 2:1369–1370
Agrawal B, Gendler SJ, Longenecker BM (1998) The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol Med Today 4:397–403
Lacunza E, Baudis M, Colussi AG, Segal-Eiras A, Croce MV, Abba MC (2010) MUC1 oncogene amplification correlates with protein overexpression in invasive breast carcinoma cells. Cancer Genet Cytogenet 201:102–110
Duffy MJ (1999) CA 15–3 and related mucins as circulating markers in breast cancer. Ann Clin Biochem 36:579–586
Duffy MJ, Shering S, Sherry F et al (2000) CA 15–3: a prognostic marker in breast cancer. Int J Biol Markers 15:330–333
Harris L, Fritsche H, Mennel R et al (2007) American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312
McLaughlin R, McGrath J, Grimes, Given HF (2000) The prognostic value of the tumor marker CA 15–3 at initial diagnosis of patients with breast cancer. Int J Biol Markers 15:340–342
Canizares F, Sola J, Perez M et al (2001) Preoperative values of CA15.3 and CEA as prognostic factors in breast cancer: a multivariate analysis. Tumor Biol 22:273–281
Gion M, Boracchi P, Dittardi R et al (2002) Prognostic role of serum CA 15-3 in 362 node-negative breast cancers. An old player for a new game. Eur J Cancer 38:1181–1188
Ebeling FG, Stieber P, Untch M et al (2002) Serum CEA and CA 15–3 as a prognostic factors in primary breast cancer. Br J Cancer 86:1217–1222
Kumpulainen EJ, Keskikuru RJ, Johansson RT (2002) Serum tumor marker CA 15–3 and stage are the two most powerful predictors of survival in primary breast cancer. Breast Cancer Res Treat 76:95–102
Molina R, Filella X, Alicarte J et al (2003) Prospective evaluation of CEA and CA15.3 in patients with locoregionale breast cancer. Anticancer Res 23:1035–1042
Duffy MJ, Duggan C, Keane R et al (2004) High preoperative CA 15–3 concentrations predict adverse outcome in node-negative and node-positive breast cancer: study of 600 patients with histologically confirmed breast cancer. Clin Chem 50:559–563
Martin A, Corte MD, Alvarez AM et al (2006) Prognostic value of pre-operative serum CA15.3 levels in breast cancer. Anticancer Res 26:3965–3972
Velaiutham S, Taib NA, Ng KL, Yoong BK, Yip CH (2008) Does the pre-operative value of serum CA15.3 correlate with survival in breast cancer? Asian Pac J Cancer Prev 9:445–448
Park BW, Oh JW, Kim JH et al (2008) Preoperative CA 15–3 and CEA serum levels as predictor for breast cancer outcomes. Ann of Oncol 19:675–681
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22:1736–1747
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Viale G, Regan MM, Maiorano E et al (2007) Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. J Clin Oncol 25:3846–3852
Viale G, Giobbie-Hurder A, Regan MM et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: Results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26:5569–5575
Rasmussen BB, Regan MM, Lykkesfeldt AE et al (2008) Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. Lancet Oncol 9:23–28
Gray RJ (1998) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 16:1141–1154
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8:551–561
Antolini L, Nam B-H, D’Agostino RB (2004) Inference on correlated discrimination measures in survival analysis: a nonparametric approach. Comm Statist Theory Methods 33:2117–2135
Davis BW, Gelber RD, Goldhirsch A et al (1986) Prognostic significance of tumor grade in clinical trials of adjuvant therapy for breast cancer with axillary lymph node metastasis. Cancer 58:2662–2670
Layfield LJ, Goldstein N, Perkinson KR, Proia AD (2003) Interlaboratory variation in results from immunohistochemical assessment of estrogen receptor status. Breast J 9:257–259
Diaz LK, Sneige N (2005) Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy. Adv Anat Pathol 12:10–19
Perez EA, Suman VJ, Davidson NE et al (2006) HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol 24:3032–3038
Mukhopadhyay P, Chakraborty S, Ponnusamy MP et al (2011) Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta 1815:224–240
Perou C, Sorlie T, Elsen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Perou AM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790–800
Viale G, Rotmensz N, Maisonneuve P et al (2009) Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat 116:317–328
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandri, M.T., Salvatici, M., Botteri, E. et al. Prognostic role of CA15.3 in 7942 patients with operable breast cancer. Breast Cancer Res Treat 132, 317–326 (2012). https://doi.org/10.1007/s10549-011-1863-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1863-x