Abstract
HER2 gene amplification and topoisomerase IIα gene (TOP2A) alteration have been associated with increased benefit from anthracycline compared to non-anthracycline containing adjuvant breast cancer chemotherapy in some but not other studies. Chromosome 17 centromere (CEP17) duplication was measured on TMAs from formalin-fixed paraffin-embedded specimens obtained from 639 of 716 premenopausal women with node positive breast cancer who received cyclophosphamide, epirubicin and fluorouracil (CEF) or cyclophosphamide, methotrexate and fluorouracil (CMF) in the randomized controlled mammary 5 (MA.5) adjuvant trial. The prognostic impact of CEP17 duplication and its interactions with treatment were studied for relapse-free survival (RFS) and overall survival (OS). Overall, CEP17 duplication was not significantly associated with RFS or OS in multivariate analysis. For patients whose tumours had normal CEP17 copy number there were no apparent benefits for CEF compared to CMF for RFS (HR 0.98; 95% CI 0.68–1.42) or OS (HR 1.10; 95% CI 0.72–1.69). For patients whose tumours had CEP17 duplication, there was significant benefit for CEF compared to CMF for RFS (HR 0.54; CI 0.33–0.89) and a trend towards significance for OS (HR 0.64; CI 0.37–1.09). The adjusted P values for interaction between treatment and CEP17 duplication were 0.09 for RFS and 0.13 for OS. This study suggests that CEP17 duplication has a borderline association with clinical responsiveness to anthracycline containing chemotherapy similar to previous results seen with HER2 amplification and TOP2A alteration in MA.5. An appropriately powered meta-analysis is required to discriminate the predictive value of these three candidate markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of previous studies associated both HER2 overexpression and amplification with a favourable tumour response to anthracycline compared to non-anthracycline containing chemotherapy [1–6], although only two of these analyses reached statistical significance [3, 6]. These observations were supported by the publication of three meta-analyses based on literature reports of published data [7–9]. However, inconsistency between studies relating to HER2 led to a broader search for an appropriate biomarker. Subsequently, as a result of the proximity of HER2 and topoisomerase IIα (TOP2A) on chromosome 17 [10, 11] it was hypothesized that TOP2A possibly represented the true target gene for anthracycline benefit. Several studies reported on association between TOP2A gene alterations and greater responsiveness to anthracyclines [10–12] again with mixed results. Knoop et al. [11] suggested that whilst HER2 status gave no predictive information with respect to the benefit of anthracycline-containing chemotherapy, TOP2A deletion or amplification predicted benefit with borderline significance. A recent article by Pritchard et al. [13] reviewed the associations of HER2 amplification/overexpression and TOP2A gene alterations from the published literature and found them inconsistent.
The potential role of TOP2A and HER2 as predictive biomarkers for anthracycline benefit was most recently addressed in two linked UK studies [14, 15]. In an analysis of 1,870 breast cancers from the linked BR9601/NEAT studies, no consistent predictive value of either HER2 or TOP2A was shown [14, 15] and in a central, individual patient meta-analysis of four trials including 1,944 patients, Di Leo et al., showed a modest and only statistically borderline predictive value for either of these biomarkers [16, 17]. These studies raise significant questions and highlight controversy regarding the association between HER2 or TOP2A gene alterations and the benefit of anthracycline-containing therapies in comparison to non-anthracycline-containing therapies in early breast cancer.
Previous studies have separately analysed, on the one hand, HER2 amplification or overexpression or TOP2A gene amplification or deletion [1–18], topoisomerase II protein (topo2α) [19] and on the other, more conventional markers of response such as proliferation [20, 21]. The piecemeal publication of results relating to different biomarkers, often in under-powered patient cohorts, has hampered efforts to discriminate between the potential associations amongst HER2 and TOP2A alterations and anthracycline therapy. This is particularly true since the majority of TOP2A amplified or deleted cases are also amplified for HER2 when assayed by FISH. Interestingly, the recent analysis of the UK National Epirubicin Adjuvant Trial (NEAT)/BR9601 not only showed no interaction between HER2 and TOP2A and an anthracycline versus non-anthracycline regimen but suggested that chromosome 17 centromere (CEP17) duplication was significantly associated with the benefit of anthracycline-containing regimens [15]. Thus, we here analyze data from the NCIC Clinical Trials Group (NCIC CTG) randomized Mammary 5 (MA.5) trial of cyclophosphamide, epirubicin and 5-fluorouracil (CEF) compared to cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as adjuvant therapy for breast cancer [22, 23] to explore the association of CEP17 duplication with the efficacy of an anthracycline versus a non-anthracycline-containing regimen.
Subjects and methods
Patients
The MA.5 study randomized 716 premenopausal women with axillary lymph node-positive breast cancer (T1–T3a, N1–N2, M0) who had completed primary breast cancer surgery no more than ten weeks before random assignment [22, 23]. Patients were accrued between 1989 and 1993 at 35 centres in Canada. The MA.5 protocol was approved by the institutional review board at each participating centre and registered as NCI-V90-0027 on cancer.gov. Written informed consent was obtained from each woman before random assignment.
Treatment regimens
The adjuvant CEF regimen consisted of six cycles of epirubicin (Pharmorubicin; Pfizer, New York, NY) 60 mg/m2 and 5-fluorouracil (5-FU) (Efudex; Valeant Pharm, Aliso Viejo, CA) 500 mg/m2, both delivered intravenously on days 1 and 8, and oral cyclophosphamide (Cytoxan; Bristol Myers Squibb, New York, NY) 75 mg/m2 daily on days 1–14. During this regimen, patients received antibiotic prophylaxis with trimethoprim–sulfamethoxazole (Septra; Glaxo, Philadelphia, PA; Merck, Whitehouse Station, NJ) 400 mg orally twice daily or ciprofloxacin (Cipro; Bayer, Berlin, Germany) 500 mg orally twice daily. The CMF regimen consisted of six cycles of methotrexate (Wyeth, formerly Lederle, Madison, NJ) 40 mg/m2 and 5-FU 600 mg/m2, both delivered intravenously on days 1 and 8, and oral cyclophosphamide 100 mg/m2 daily on days 1–14.
Specimen collection
Representative formalin-fixed, paraffin-embedded (FFPE) tumour blocks from the primary surgical specimen were retrospectively requested for each woman enrolled in the study. For this analysis, pathologists were asked to submit a representative FFPE block of tumour tissue from each woman, or if tumour blocks were unavailable, 20.4-μm unstained sections, to the central office of the NCIC CTG. Paraffin blocks were stored at room temperature, and unstained sections were kept at 4°C. Samples were identified only by an identification number assigned to each patient at randomization. A stained section of each tumour sample was prepared from blocks or slides to confirm the diagnosis and to identify representative tumour areas for microdissection. Further 4 μm sections were obtained for immunohistochemical analysis and fluorescence in situ hybridization (FISH). Assay results were reported to the NCIC CTG central office, where the statistical analysis was performed.
Measurements of HER2 and TOP2A amplifications and CH17 CEP centromere duplication
HER2 and TOP2A amplifications and deletions were measured by FISH as described in our previous studies [12]. The FISH probes used were the PathVysion HER2 DNA probe kit and the Locus Specific Identifier (LSI) TOP2A/CEP17 probe kit (both from Vysis-Abbott, Downer’s Grove, Illinois, USA). Slides were prepared according to the manufacturer’s instructions for the paraffin sections. Sections were analysed using a Leica DMBRX epifluorescence microscope (Bannockburn, IL) equipped with filters for the separate detection of 4′,6-diamidino-2-phenylindole, spectrum green, and spectrum orange and with a triple bandpass filter for simultaneous detection of the three signals. The number of signals representing the gene of interest (TOP2A or HER2) and the number of chromosome 17 centromeres present in each cell were recorded for a minimum of 60 nuclei per case. Images were captured by a charge-coupled device camera using software from Applied Imaging (Santa Clara, CA). The ratio of the signal of interest per chromosome 17 centromere was calculated for each sample. A ratio of HER2 to CEP17 of 2.0 or more was considered to indicate HER2 amplification. A tumour was considered to have amplified TOP2A if the TOP2A:CEP17P ratio was 2.0 or greater; to have deleted TOP2A if the ratio was 0.8 or less; and to have normal TOP2A copy number if the ratio was between 0.8 and 2. “Altered” TOP2A was a combined category that included both patients with deleted and with amplified TOP2A. CEP17 duplication copy number results were collected for all cells with a minimum of two C17 signals/cell and CEP17 duplication defined as >2.25 mean observed copies/cell [24, 25] as this cut-off has been specifically validated for the scoring approach used here.
Statistics
Relapse-free survival (RFS), defined as time from randomization to first recurrence, and overall survival (OS), defined as time from randomization to death from any cause, were two outcomes of this study. The Kaplan–Meier method was used to estimate the RFS and OS at 5 years and associated confidence intervals. Univariate Cox proportional hazard model including only one single factor or multivariate Cox proportional hazard model adjusting for age (≥50, <50); number of positive nodes (≤3, ≥4); oestrogen receptor (ER) protein levels from the clinical data base of the MA.5 trial, i.e., local ER measurements from each centre (≥10, <10 fm protein/ml); surgical type (total vs. partial mastectomy); tumour size (T1, T2, T3), tumour grade (1, 2, 3, using the Elston & Ellis grading system [24], performed by central review on whole sections) and HER2 (amplified or not amplified) and TOP2A (altered or normal) as measured by FISH was used to obtain hazard ratios for relapse or death.
Results
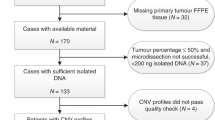
There were no apparent differences in patient characteristics between the overall trial populations and the populations included in this TMA study (Table 1) (consort diagram: Fig. 1). Of 628 patients included in this study, 332 (53%) relapsed and 250 (40%) died during the period of follow-up.
CEP17 duplication
CEP17 duplication as defined above was observed in 253/628 (40.3%) of cases included in the analysis. No significant association was observed between HER2 gene amplification (observed in 24.4% of cases) and CEP17 duplication (P = 0.95). Cases with CEP17 duplication were equally distributed between HER2 amplified (40.2%) and non-amplified (40.5%) cases. Only tumour grade was significantly associated with CEP17 duplication (P = 0.006; Table 1).
Association with RFS and OS
In univariate analysis, CEP17 duplication was not significantly associated with RFS (HR 1.19, 95% CI 0.96–1.49, P = 0.12; Fig. 1a) but its association with OS was significant (HR = 1.33, 95% CI 1.04–1.76 and P = 0.03; Fig. 1b) (Table 2). In Cox multiple regression models including the following covariates: CEP17 status; HER2 status; TOP2A status; ER status; age; grade; nodal status; tumour size; surgery and treatment (CEF vs. CMF): tumour size, nodal status grade and surgery types were significantly associated with both RFS and OS. None of the three biomarkers (HER2, TOP2A or CEP17 status) was found significantly associated with either RFS or OS after adjusting for other variables (Table 3).
a RFS by CEP17 status, b overall survival by CEP17 status, c RFS by treatment for those with normal CEP17 copy number, d RFS by treatment for those with CEP17 duplication, e overall survival by treatment for those with normal CEP17 copy number and f overall survival by treatment for those with CEP17 duplication
Treatment by marker interactions
For tumours with normal CEP17 copy number there were no apparent benefits for CEF compared to CMF for RFS (HR 0.98 with 95% CI 0.68–1.42, P = 0.93; Fig. 1c) or OS (HR 1.10 with 95% CI 0.72–1.69, P = 0.66; Fig. 1e). Conversely, for tumours exhibiting CEP17 duplication, the relative risks of relapse and death for patients receiving CEF rather than CMF were 0.54 (95% CI 0.33–0.89; P = 0.02, Fig. 1d) and 0.64 (95% CI 0.37–1.09, P = 0.10; Fig. 1f), respectively (Table 4).
In univariate analysis, CEP17 duplication was associated with a significantly increased benefit from CEF compared to CMF (Table 5) for both RFS and OS with treatment by marker interactions (HR 0.59 with 95% CI 0.37–0.92, P = 0.02) and (HR 0.61 with 95% CI 0.37–1.01, P = 0.05), respectively. Multivariate Cox regression analyses adjusting for age, nodal status, ER status, grade, tumour size, surgery type, HER2 status and TOP2A status, showed a non-significant trend for a treatment by CEP17 interaction with respect to RFS (HR 0.59 with 95% CI 0.32–1.08, P = 0.09) but not with OS (HR 0.60 with 95% CI 0.31–1.12, P = 0.13) (Table 5). In contrast, in multivariate regression analyses using the Cox model, treatment by HER2 interaction was borderline significant for both RFS (HR 0.58 with 95% CI 0.33–0.99, P = 0.05) and OS (HR 0.57 with 95% CI 0.31–1.02, P = 0.06) and treatment by TOP2A interaction showed a trend towards significance for RFS (HR 0.53 with 95% CI 0.26–1.09, P = 0.09) and significant interaction with OS (HR 0.38 with 95% CI 0.17–0.85, P = 0.02) (Table 5).
HER2, TOP2A and CEP17 duplication sub-group analysis
Exploratory sub-group analyses were performed by combining HER2 amplification status or TOP2A alteration status with CEP17 duplication status (Table 6). All cases with CEP17 duplication exhibited improved outcome from CEF compared to CMF (RFS or OS). Some evidence of an effect of HER2 or TOP2A was seen in the exploratory analyses. Cases with none of HER2 amplification, TOP2A alteration or CEP17 duplication trended towards increased benefit from CMF rather than CEF.
Discussion
We have previously reported conflicting results on the role of type I receptor kinases as biomarkers for anthracycline response in two separate trials in early breast cancer (MA5 and NEAT/BR9601) [3, 12, 14, 15]. In MA5, HER2-amplified tumours and TOP2A gene-altered tumours were characterised by increased benefit from inclusion of anthracyclines [3, 12], whilst in NEAT/BR9601 HER2 non-amplified (and HER1/3 low expressing) tumours appeared to derive enhanced benefit from anthracycline-based chemotherapy [14, 15]. In spite of differences in chemotherapy regimens and patient characteristics between these two studies, similar molecular methods were employed in both centres for the analysis of patient samples. Since, the observed differences between these studies provided no rational explanation for differences in results, we explored alternative scientific hypotheses which might unify the results for these two studies.
Following an observation by Reinholz et al. [25] suggesting paradoxically good outcome for patients with CEP17 duplication in tumours treated with AC-T we examined the relationship between CEP17 duplication and anthracycline response in each of our studies. Within the MA.5 trial, patients whose tumours exhibit CEP17 duplication showed a greater than 46% reduction in risk of relapse (either local or distant) and 36% in risk of death when treated with CEF instead of CMF (Table 4, Fig. 1c) consistent with results from the NEAT/BR9601 study [15]. No apparent benefit from treatment with anthracyclines was observed in tumours with normal CEP17 copy numbers (Fig. 1c). A significant treatment by marker interaction for both RFS (P = 0.02) and OS (P = 0.05) was observed in univariate analysis, although only a trend towards significance was observed for RFS after adjustment for other variables.
These results, linking CEP17 duplication to benefit from CEF compared to CMF show similarity to those obtained from an analysis of the two UK trials BR9601 and NEAT [14, 15] suggesting that the measurement of CEP17 copy numbers may have provided a unifying biomarker for the prediction of benefit from anthracycline containing versus non-anthracycline-containing regimens. The NCIC CTG, UK (BR9601) and Scottish trial (NEAT) groups plan to use this marker in an individual patient meta-analysis with the same Danish and Belgian groups with whom we have previously examined the role of HER2 amplification and TOP2A gene alterations [16, 17].
Attempts to explore the interaction of HER2 amplification status, TOP2A alteration status and CEP17 duplication status with outcome in the two treatment groups were problematic mainly because of ever-diminishing sample size. Of the 639 patients studied in this CEP17 correlative analysis only 395 were available to deal with a multivariate analysis when all three potentially predictive factors were included, mainly because TOP2A data was available on only 438 women.
The rationale for an association between CEP17 duplication and anthracycline benefit is not intuitively obvious. The way in which we have measured CEP17 duplication may indicate changes including imbalanced translocations, sub-chromosomal amplification or deletion or whole chromosome genome duplication. Most previous studies using FISH interpreted duplication of CEP17 as chromosomal polysomy. The CEP17 marker however does not identify polysomy, therefore we have used the more accurate description of CEP17 duplication to describe this finding. Our approach does not invalidate previous definitions of HER2 amplification since these definitions are also based on the ratio of the HER2 gene and the CEP17 centromere irrespective of the underlying chromosomal defect.
Although, our observation that CEP17 duplication is associated with benefit from anthracycline may offer a practical clinical approach to the selection of patients for such therapy, it is not clear what insight it provides into the possible mechanisms of action. Recent articles [26, 27] suggest that chromosomal 17 polysomy is far less common in early breast cancer than was previously suggested and that CEP17 duplication does not simply indicate polysomy or tumour anuploidy but also identifies cancers with sub-chromosomal duplication or amplification of the CEP17 region which is close to the HER2 amplicon. We cannot tell at the moment which of these different gene abnormalities may be associated with the underlying mechanism of anthracycline sensitivity. Further research with comparative genomic hybridization (CGH) or expression array analysis may be helpful in clarifying the potential mechanisms underlying these clinical/pathogenic observations. These mechanistic explanations should be explored to prepare more informed approaches to the future use of DNA damaging agents in populations who may be resistant to anthracyclines as well as guiding anthracycline usage.
In any case, we conclude that, since some other trials did not find HER2 amplification or TOP2A alteration to be predictive of a differential benefit from CEF compared to CMF, CEP17 duplication may be a more consistent marker. Results from other trials and from our planned individual patient meta-analysis may clarify this matter if enough patients and their tumour markers can be included.
References
Di Leo A, Gancberg D, Larsimont D et al (2002) HER-2 amplification and topoisomerase II alpha gene aberrations as predictive markers in node positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clin Cancer Res 8:1107–1116
Di Leo A, Larsimont D, Beauduim M (2001) Her-2 and topoisomerase II alpha as predictive markers in a population of node-positive breast cancer patients randomly treated with adjuvant CMF or epirubicin plus cyclophosphamide. Ann Oncol 12:1081–1089
Pritchard KI, Shepherd LE, O’Malley FA et al (2006) HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354:2103–2111
Muss HB, Thor AD, Berry DA et al (1994) C-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. New Engl J Med 330:1260–1266
Paik S, Bryant J, Tan-Chiu E et al (2000) HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National surgical adjuvant breast and bowel project protocol B-15. J Natl Cancer Inst 92:1991–1998
Paik S, Bryant J, Park C et al (1998) ErbB-2 and response to doxorubicin in patients with axillary lymph node positive, hormone receptor negative breast cancer. J Natl Cancer Inst 90:1361–1370
Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P (2008) HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 100:14–20
Dhesy-Thind B, Pritchard KI, Messersmith H et al (2008) Her-2/neu in systemic therapy for women with breast cancer: a systemic review. Breast Cancer Res Treat 109:209–229
De Laurentiis M, Arpino G, Massarelli E et al (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748
Slamon DJ, Mackey J, Crown J et al (2007) Role of anthracycline-based therapy in the adjuvant treatment of breast cancer: efficacy analyses determined by molecular subtypes of the disease [abstract]. Breast Cancer Res Treat 106:112 (Abstract 13)
Knoop AS, Knudsen H, Balslev E et al (2005) Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 23:7483–7490
O’Malley F, Chia S, Tu D et al (2009) Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst 101:644–650
Pritchard KI, Messersmith H, Elavathil L et al (2008) Her-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol 26:1–9
Bartlett JMS, Munro A, Cameron DA et al (2008) Type I receptor tyrosine kinase profiles identify patients with enhanced benefit from anthracyclines in the BR9601 adjuvant breast cancer chemotherapy trial. J Clin Oncol 26:5027–5035
Bartlett JMS, Munro A, Dunn JA et al (2010) Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol 11:266–274
Di Leo A, Desmedt C, Bartlett JMS et al (2010) Final results of a meta analysis testing HER2 and topoisomerase II genes as predictors of incremental benefit from anthracyclines in breast cancer [abstract]. J Clin Oncol 28:72S
Di Leo A, Isola J, Piette F et al (2008) A meta-analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase II alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy [abstract]. Breast Cancer Res Treat 107:24s (Abstract 705)
Jarvinen TA, Tanner M, Rantanen V et al (2000) Amplification and deletion of topoisomerase II alpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 156:839–847
O’Malley FA, Chia S, Tu D et al (2011) Topoisomerase II alpha protein and responsiveness of breast cancer to adjuvant chemotherapy with CEF compared to CMF in the NCIC CTG randomized MA.5 adjuvant trial. Breast Cancer Res Treat 128:1511–1515
Jarvinen TA, Kononen J, Peltohuikko M, Isola J (1996) Expression of topoisomerase II alpha is associated with rapid cell proliferation, aneuploidy, and c-erbB2 overexpression in breast cancer. Am J Pathol 148:2073–2082
Bartlett JMS, Ellis IO, Dowsett M et al (2007) Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor negative, but with grade in those with ER positive early stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol 25:4423–4430
Levine MN, Bramwell VH, Pritchard KI et al (1998) A randomized trial of cyclophosphamide, epirubicin, fluorouracil chemotherapy compared with cyclophosphamide, methrotrexate, fluorouracil in premenopausal women with node positive breast cancer. J Clin Oncol 16:2651–2658
Levine MN, Pritchard KI, Bramwell VH et al (2005) A randomized trial comparing CEF to CMF in premenopausal women with node positive breast cancer: update of NCIC CTG MA.5. J Clin Oncol 23:5166–5170
Elston CW (1991) Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Reinholz MM, Jenkins RB, Hillman DW et al (2007) The clinical significance of poly 17 in the HER2 and N98431 intergroup adjuvant trastuzumab trial [abstract]. Breast Cancer Res Treat 107:S11 (Abstract 36)
Yeh I-T, Martin MA, Robetorye RS et al (2009) Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol 22:1169–1175
Marchio C, Lambros MB, Gugliotta P et al (2009) Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 219:16–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pritchard, K.I., Munro, A., O’Malley, F.P. et al. Chromosome 17 centromere (CEP17) duplication as a predictor of anthracycline response: evidence from the NCIC Clinical Trials Group (NCIC CTG) MA.5 Trial. Breast Cancer Res Treat 131, 541–551 (2012). https://doi.org/10.1007/s10549-011-1840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1840-4