Abstract
Surgical margin status after first breast-conserving surgery (BCS) is used as a quality indicator of breast cancer care in the Netherlands. The aim is to describe the variation in surgical margin status between hospitals. 7,345 patients with DCIS or invasive cancer (T1-2,N0-1,M0) diagnosed between July 1, 2008, and June 30, 2009, who underwent BCS as first surgery, were selected from the Netherlands Cancer Registry. Patients were treated in 96 hospitals. Maximum target values were 30% ‘focally positive’ or ‘more than focally positive’ for DCIS and 10% ‘more than focally positive’ for invasive carcinoma. Results per hospital are presented in funnel plots. For invasive carcinoma, multivariate logistic regression was used to adjust for case mix. Overall 28.5% (95% CI: 25.5–31.4%) of DCIS and 9.1% (95% CI: 8.4–9.8%) of invasive carcinoma had positive margins. Variation between hospitals was substantial. 6 and 10 hospitals, respectively, for DCIS and invasive cancer showed percentages above the upper limit of agreement. Case mix correction led to significant different conclusions for 5 hospitals. After case mix correction, 10 hospitals showed significant higher rates, while 7 hospitals showed significant lower rates. High rates were not related to breast cancer patient volume or type of hospital (teaching vs. non-teaching). Higher rates were related to hospitals where the policy is to aim for BCS instead of mastectomy. The overall percentage of positive margins in the Netherlands is within the predefined targets. The variation between hospitals is substantial but can be largely explained by coincidence. Case mix correction leads to relevant shifts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast-conserving surgery (BCS) is common practice in the treatment of early breast cancer patients. BCS with additional radiotherapy leads to better cosmetic results with equal long-term disease-free and overall survival compared to mastectomy. In the Netherlands, over the period 2003–2006, more than 21,000 patients with early breast cancer underwent BCS (63% of stage I and 41% stage II breast cancer) [1].
Surgeons aim to obtain a radical excision of the tumor in BCS, since so-called tumor-free margins result in the best local control. The size of the lump is a balance between cosmetic aspects and completeness of surgery: A wider excision than needed leads to a worse cosmetic result, but a too narrow excision may leave residual tumor tissue. Incomplete resections lead to additional surgical procedures (either BCS or mastectomy), which implicate extra burden on the patient and extra costs.
The surgical margins of the excised lump of BCS are examined by a pathologist according to protocols. The amount of cancer that could have been left in the breast is estimated. Based on Dutch breast cancer treatment guidelines, the pathologist distinguishes ‘clear’ margins (tumor not touching the inked surface of the lump), ‘focally positive’ margins (one or two foci of tumor touching ink; less than 4 mm), and ‘more than focally positive’ margins. Margin status determines the further steps needed in adequate local treatment. All patients with BCS (DCIS and invasive cancer) receive additional radiotherapy, but the indication for re-excision varies for patients with DCIS or invasive cancer. Patients with DCIS undergo re-excision in case of ‘focally positive’ or ‘more than focally positive’ margins, while patients with invasive cancer undergo re-excision only in case of ‘more than focally positive’ margins. In case of ‘focally positive’ margins, local control is (in most cases) supposed to be achieved by more radiotherapy including a boost dose on the tumor bed [2].
The percentage of patients with positive margins is used as a quality indicator of breast cancer care that all hospitals have to report to the Dutch Health Care Inspectorate annually since 2007 [3]. The target value to be achieved is 10% for DCIS and invasive carcinoma combined. The current Dutch breast cancer treatment guidelines state that surgeons should strive for a maximum of 20% ‘more than focally positive’ margins in patients with invasive carcinoma and 30% ‘focally positive’ or ‘more than focally positive’ margins in patients with DCIS [2].
Apart from the discussion on the agreed target value, there is the issue of comparability of data provided by hospitals due to registration artifacts (such as case selection and the multi-interpretability of ‘positive margin’ when combining DCIS and invasive tumors) and case mix differences between hospitals [4, 5].
In 2008, the Netherlands Cancer Registry (NCR) started the collection of information on margin status after the first BCS aiming to provide comparable and population-based data on all breast cancer patients in the Netherlands, including information on case mix. This study describes the variation between hospitals in surgical margins after first breast-conserving surgery (BCS) in patients with DCIS or early breast cancer in the Netherlands.
Methods
Study population
All female early breast cancer patients (DCIS and invasive carcinoma T1-2, N0-1, M0; LCIS not included) who underwent BCS in the period July 1, 2008, to June 30, 2009, were selected from the Netherlands Cancer Registry (NCR). Patients who received neo-adjuvant systemic therapy were excluded.
The NCR is a population-based cancer registry, collecting incidence data on national level. PALGA, the Dutch network and registry of pathology, notifies the NCR of all newly diagnosed malignancies. Following this notification, trained NCR personnel collect data on diagnosis, staging, and treatment from hospital records, including pathology and surgery reports. Primary treatment is coded in sequence of administration, and patients are staged according to the TNM system of the International Union Against Cancer [6].
We extracted information on patient characteristics (age), tumor characteristics (histological subtype, grade, localization, multifocality, TNM stage, tumor size), and treatment characteristics (neo-adjuvant treatment, surgical treatment, radiotherapy, surgical margin status after first BCS and hospital of treatment).
Classification of surgical margins
Coding of the surgical margin status was based on the most recent Dutch diagnostic and treatment guideline for breast cancer [2]. The guideline defines how pathologists should assess surgical margin status after breast-conserving surgery (BCS) and subsequently what information should be included in their report. The classification of surgical margins in the Dutch breast cancer guideline defines 3 categories: clear surgical margins (no tumor cells in the inked surface of the resection), ‘focally positive’ margins (tumor in a limited area of the inked surface, i.e., one or two foci of tumor, maximum of 4 mm), and ‘more than focally positive’ margins. In records with unclear or missing information, the margin status was coded as ‘unknown’. The assessment of tissue from following procedures was not used in the classification of the margin status after the first BCS.
Classification of hospitals
Classification of hospitals was based on the hospital where surgery was performed. All Dutch hospitals (96) were included. Two types of hospitals were defined: 42 non-teaching hospitals and 54 teaching or academic hospitals (including one specialized oncology center). Hospital volume was based on the number of breast cancer patients with BCS as the first surgery, and two groups were defined: less than 50 BCS/y (33 hospitals) and 50 BCS/y or more (63 hospitals). The percentage of BCS in a hospital was calculated by dividing the number of patients who underwent a BCS as first surgery by the total number of patients with DCIS or invasive carcinoma T1-2,N0-1,M0 who received surgery in that hospital. Hospitals were categorized into two groups; less than 70% BCS (66 hospitals) and 70% BCS or more (30 hospitals).
Quality indicator targets
All results are presented separately for DCIS and invasive breast cancer. Patients with invasive tumor and DCIS component(s) are included in the invasive group. For invasive cancer, positive margins were those classified as ‘more than focally positive’ with a target value of 10%, based on the targets set by the Dutch Health Care Inspectorate. For DCIS, we defined positive margins as margins that were classified by pathologists as ‘focally positive’ or ‘more than focally positive’ with a target value of 30% based on the breast cancer guideline.
Statistical analyses
The proportions of positive margins per hospital are presented in funnel plots. The funnel plot presents the target with its 95% confidence limit that varies in relation to the population size [7]. We also computed the number of hospitals with percentage of positive margins outside the limits of agreement at various target values (10, 20, and 30% for DCIS; 10 and 20% for invasive cancer). Patients with unknown surgical margins (2.7%) were excluded in univariate and multivariate analyses.
For case mix correction in invasive cancer, we first selected risk factors of positive margins using univariate logistic regression. These factors were based on literature and included age, tumor size, nodal status, multifocality, and histological subtype [8–11]. Significant factors were included in a multivariate logistic regression model to determine the mutually independent factors. Subsequently, the obtained coefficients were used to predict for each individual the risk of positive margins based on her set of risk factors. Next, for each hospital, the expected percentage of patients with positive margins was assessed based on their specific case mix (E). Then, the observed percentage (O) was divided by the expected value (E) and multiplied by the overall mean (9.1% for invasive cancer, O/E * mean) to obtain the case mix–adjusted percentages. These for case mix–adjusted percentages of patients with positive margins are presented per hospital in a funnel plot.
To explore the characteristics of hospitals with percentages above and under the limits of 10% positive margins, these hospitals were compared to the others on type of hospital, the number of breast cancer patients with BCS as first surgery per year, and the percentage of breast cancer patients who received BCS as first surgery. Differences were tested using Fisher’s exact test.
Analyses were performed in STATA and SPSS (multivariate logistic regression).
Results
A total of 7,345 patients who underwent BCS were identified in the period July 1, 2008, and June 30, 2009, in the Netherlands. BCS was performed in all 96 hospitals. Mean age at diagnosis was 59 years. 945 patients were diagnosed with DCIS (12.9%). 82% of all lesions were of ductal type, and more than 75% of women had lymph node-negative disease (Table 1). The surgical margin status was known for 7,146 patients (97.3%) (Table 2). Overall, 9.5% of all resections margins were classified as ‘focally positive’, while another 10.0% showed ‘more than focally positive’ margins. These percentages were higher in patients diagnosed with DCIS than in patients with invasive cancer (Table 2). The percentage of women with ‘unknown’ margin status varied substantially between hospitals (0–9.4%, data not shown).
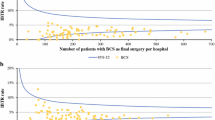
The proportion of patients with positive margins varied substantially by hospital. For DCIS, the mean proportion ‘focally positive’ or ‘more than focally positive’ margins was 27% and ranged between 0 and 100%. Most of this variation could be due to coincidence as a result of the low number of DCIS in each hospital (1–34 patients). The results of 88 hospitals (92%) fit within the limits of agreement of the proposed 30% target (Fig. 1): 6 hospitals showed higher and 2 lower percentages of positive margins. If a target of 20 or 10% is used, 17 and 43 hospitals, respectively, would fall outside the agreement limits (all too high).
For invasive tumors, the mean proportion of ‘more than focally positive’ margins was 9.1%, with less variation between hospitals (0–30%), due to more stable estimates based on larger numbers of cases operated per hospital (between 10 and 173 patients). For 80 hospitals (87%), results fit within the limits of agreement at the proposed target of 10% ‘more than focally positive’ margins. 10 hospitals showed statistically significant higher percentages, while 6 hospitals showed statistically significant lower percentages (Fig. 2). When the target of 20% was applied, no hospitals had higher proportions than the limits of agreement around this target, and 50 hospitals (52%) had significant lower percentages.
None of the hospitals had higher proportions of positive margins for both DCIS and invasive tumors.
Risk factors for positive margins and correction for case mix
For DCIS, multifocality was associated with a significant higher rate of positive margins (52% vs. 26%) (Table 3). Case mix correction for the percentage of positive margins in DCIS treatment was not possible due to limited numbers per hospital.
For invasive tumors, characteristics that were associated with higher rates of positive margins in univariate analyses were younger age, larger tumors, nodal involvement, multifocality, and a lobular or ductolobular histological type (Table 3). Multivariate results are shown in Table 4. The obtained coefficients from the multivariate analyses were used to perform case mix correction for invasive cancers. This slightly affected the observed estimates. In 5 hospitals (8.3%), case mix correction altered the conclusion on whether the proportion of positive margin status was outside the limits of agreement. For 3 hospitals, the adjusted percentage was lower than the uncorrected (two hospitals into and one under the limits of agreement), and for 2 hospitals, the adjusted percentage was higher after case mix correction (both above the limit of agreement) (Fig. 3). The net result is that 10 hospitals showed positive margin rates significantly higher than 10%, while 7 hospitals showed significant lower rates.
Hospitals with significant higher positive margin rates frequently had a high percentage first BCS (6 hospitals out of 33 compared to 6 out of 66; P = 0.05) but did not differ on other hospital characteristics (type of hospital and number of BCS per year). Hospitals with significant lower positive margin rates treated more often over 50 patients with BCS per year (7 hospitals out of 63 compared to 0 out of 33; P = 0.05) but did not differ on other characteristics.
Discussion
The percentage of breast cancer patients with positive surgical margins after a first breast-conserving surgery in the Netherlands is approximately 30% for women with DCIS and 10% for early invasive cancers. These patients need a second surgical intervention, leading to additional costs, extra burden on the patient, and a poorer cosmetic result. We found substantial differences between hospitals that were larger for DCIS than for invasive cancer. The differences were largely attributed to coincidence due to relatively low numbers of breast cancer cases treated in each hospital. However, taken into account random variation and case mix correction for invasive cancer, the percentages of positive margins were above 30% for DCIS and 10% for invasive cancers for 6 and 10 hospitals, respectively. None of the hospitals had a percentage of positive margins above the limit of agreement for both DCIS and invasive cancers.
Questions have been raised about the comparability of data on surgical margins after BCS between hospitals [3–5]. Our study has the advantage that data were collected by trained personnel of the cancer registry directly from the medical records of patients. Personnel was instructed on coding, following the Dutch diagnosis and treatment guidelines for breast cancer, with clear instructions on how to code in case of inconsistent information, additional information from re-excisions, and the inclusion and exclusion of cases such as LCIS. However, if the source information is not comparable, for example due to differences in scoring by pathologists, this cannot be solved by trained data collectors. We observed considerable variation in percentage of cases with missing data on margin status between hospitals, ranging from 0 up to 9.5%. Unknown margin status was coded for various reasons; ‘true unknown’ (tissue could not be assessed, for instance because it was presented in various lumps, without adequate marking) or unclear definition by the pathologist. All cases with unknown margin status were excluded in further analyses. However, this information would be of additional value when assessing the quality of care of a hospital.
In our study, we lack information on the presence of a DCIS component in the case of invasive breast cancer. Our study showed that DCIS leads more often to incomplete resections than invasive tumors. Therefore, adding this information to the case mix adjustment could improve the comparability of hospitals. Furthermore, a recent study showed that prediction models for margin status in DCIS had very large unexplained differences between surgeons, while controlling for detailed clinical and nonclinical factors [12]. In our study, we were not able to assess outcome on the level of individual surgeons.
Definition of margin status
Comparing data between hospitals asks for unambiguous definitions. Firstly, the definition used by the Dutch Health Care Inspectorate is problematic because it combines DCIS and invasive tumors. Complete resection rates of these lesions differ considerably even in highly qualified surgeons, and this is due to the specific biological behavior of DCIS. The observed result on hospital level would be highly determined by the proportion of DCIS diagnosed and treated in that hospital. The quality indicator should therefore be computed separately for invasive and non-invasive lesions.
Secondly, the definition of ‘positive margins’ differs between DCIS and invasive tumors. The focus of this quality indicator is the proportion of patients that needs to undergo further surgery after a first BCS. The clinical significance of the need for re-excision lies in the subsequent local recurrence and mortality rates, which is also influenced by tumor and patient characteristics [13]. The criteria determining the indication for re-excision differ between DCIS and invasive cancer. For invasive cancer, the clinical importance of re-excising tumors with only focally involved margins in invasive breast cancer is disputable [11, 13–17]. The Dutch treatment guideline (2008) advises only re-excision in patients with ‘more than focally positive’ margins. Hence, the latter definition was used to classify the proportion of patients with invasive cancer having a ‘positive margin’. In contrast, for DCIS, ‘focally positive margins’ are not acceptable and associated with a risk of local recurrence. In current literature, the most accepted definition for true clear margins for DCIS is a margin threshold of 2 mm between the lesion and the inked surface [11, 18]. The Dutch treatment guideline advises to re-excise all patients with tumor in the inked surface and to strive for a macroscopic margin threshold of 10 mm at surgery. In our study, we did not collect data on the margin threshold but used the presence of tumor in the resection surface (either focally or more than focally) as the quality indicator ‘positive margin’.
Funnel plots in interpreting hospital data
The funnel plots clearly show the large influence of randomness on the estimates when these are based on small numbers of patients: The confidence interval around the target value becomes very wide. In case of small numbers, only extreme deviations of the target will be detected as ‘abnormal’, and a large variation of measured values will fit within the confidence intervals. As such, in itself, surprising values have to be accepted as ‘in agreement with the target value’. Conversely, funnel plots also show the inappropriateness of using a crude value of 10, 20, or even 30% as a quality parameter, for a lot of incorrect accusations to hospitals will be the consequence if the 95% limits of agreement are not taken into account. This may cause hospitals and individual surgeons taking wider excisions or conducting more mastectomies to strive for much lower percentages, which would be an undesired side effect of a quality indicator that focuses on optimizing the current use of BCS.
Risk factors for positive margins and case mix correction
When comparing surgical care between hospitals, case mix correction is important to improve the comparability of data. Hospitals with less favorable patient populations improve after case mix adjustment. Univariate logistic regression showed that age, tumor size, nodal status, multifocality, and histological subtype influence the risk of positive margins. We adjusted for these characteristics through case mix correction for invasive tumors.
In 5 hospitals, the case mix adjustment led to a different conclusion on whether the observed outcome was within the limits of agreement. This demonstrates the relevance of adjustment for case mix factors. However, case mix correction requires data analysis on record level for all hospitals combined. With current methods of generating data for quality indicators by hospitals themselves, it is not possible to introduce case mix correction in the Dutch system. This calls for an independent organization, like the cancer registry, to collect and analyze these data and adjust for case mix.
The hospitals scoring above the limit of agreement had more frequent a high percentage of first BCS (≥70%). This might indicate differences in technical approach of surgeons in these hospitals. Hospitals scoring under the limit of agreement were all hospitals treating more than 50 patients with BCS per year. Both findings were borderline significant (P = 0.05). Further research is needed, in which information on the level of individual surgeons may add valuable information.
Combining quality indicators
The clinical relevance of this quality indicator lies in optimizing the quality of BCS by minimizing additional costs due to re-operation, minimizing burden on the patient, and improving cosmetic result. Others have suggested using re-resection rates or the number of operations needed for removal of the tumor as a quality indicator [19, 20]. Also, for understanding the treatment results on hospital level, we should have insight into the additional treatment for patients with positive margins [12, 21]. Combining these indicators would give a more comprehensive insight into adequate treatment.
We conclude that quality-of-care outcomes on hospital level should be interpreted separately for DCIS and invasive tumors. In addition, the limits of agreement should be taken into consideration, for example by using funnel plots. Lastly, results without case mix correction should be interpreted with caution.
References
van Steenbergen LN, van de Poll-Franse LV, Wouters MW, Jansen-Landheer ML, Coebergh JW, Struikmans H et al (2010) Variation in management of early breast cancer in the Netherlands, 2003–2006. Eur J Surg Oncol 36(Suppl 1):S36–S43
National Breast Cancer Organization of the Netherlands. Guideline breast cancer. http://www.oncoline.nl. Accessed 16 June 2011
Het resultaat telt 2008 (2009) The Hague: Dutch health care inspectorate
Gooiker GA, Veerbeek L, van der Geest LG, Stijnen T, Dekker JW, Nortier JW et al (2010) The quality indicator ‘tumour positive surgical margin following breast-conserving surgery’ does not provide transparent insight into care. Ned Tijdschr Geneeskd 154:A1142
Vles WJ (2009) Schone Schijn; Slordige data-interpretatie vloert betrouwbaarheid prestatie-indicator. Medisch Contact 2008(33/34):1354–1356
TNM Classification of Malignant Tumours (2002) 6th edn. UICC, Geneva
Spiegelhalter DJ (2005) Funnel plots for comparing institutional performance. Statist Med 24:1185–1202
Cabioglu N, Hunt KK, Sahin AA, Kuerer HM, Babiera GV, Singletary SE et al (2007) Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol 14(4):1458–1471
Kurniawan ED, Wong MH, Windle I, Rose A, Mou A, Buchanan M et al (2008) Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol 15(9):2542–2549
Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S et al (2009) The relationship between surgical factors and margin status after breast-conservation surgery for early stage breast cancer. Am J Surg 197(6):740–746
Luini A, Rososchansky J, Gatti G, Zurrida S, Caldarella P, Viale G et al (2009) The surgical margin status after breast-conserving surgery: discussion of an open issue. Breast Cancer Res Treat 113(2):397–402
Dick AW, Sorbero MS, Ahrendt GM, Hayman JA, Gold HT, Schiffhauer L et al (2011)Comparative effectiveness of Ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst 103(2):92–104
Morrow M (2010) Trends in the surgical treatment of breast cancer. Breast J 16(Suppl 1):S17–S19
Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M (2010) What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol 17(2):558–563
Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME et al (2010) Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 46(18):3219–3232
Kaufmann M, Morrow M, von MG, Harris JR (2010) Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer 116(5):1184–1191
Zavagno G, Goldin E, Mencarelli R, Capitanio G, Del BP, Marconato R et al (2008) Role of resection margins in patients treated with breast conservation surgery. Cancer 112(9):1923–1931
Dunne C, Burke JP, Morrow M, Kell MR (2009) Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol 27(10):1615–1620
Del Turco MR, Ponti A, Bick U, Biganzoli L, Cserni G, Cutuli B et al (2010) Quality indicators in breast cancer care. Eur J Cancer 46(13):2344–2356
Talsma AK, Reedijk AM, Damhuis RA, Westenend PJ, Vles WJ (2011) Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch hospitals. Eur J Surg Oncol 37(4):357–363
Virnig BA, Tuttle TM (2011) Random physician effect and comparative effectiveness of treatment for ductal carcinoma in situ. J Natl Cancer Inst 103(2):81–82
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Heiden-van der Loo, M., de Munck, L., Visser, O. et al. Variation between hospitals in surgical margins after first breast-conserving surgery in the Netherlands. Breast Cancer Res Treat 131, 691–698 (2012). https://doi.org/10.1007/s10549-011-1809-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1809-3