Abstract
The identification of women with early-stage breast cancer who will develop distant metastasis may improve clinical management. The transcriptional regulator Enhancer of Zeste-2 (EZH2) is overexpressed in invasive breast carcinoma compared with benign breast tissues, with maximal expression in breast cancer metastasis. In this article, our purpose was to investigate the performance of EZH2 protein detection as a predictor of metastasis in women with early-stage breast cancer, which is unknown. We developed a cohort of 480 women with stage I–IIA breast cancer diagnosed between 1996 and 2002 and recorded detailed sociodemographic, clinical, and pathological information. Tumors were histologically characterized and arrayed in tissue microarrays containing 1,443 samples. The nuclear EZH2 expression was investigated by immunohistochemistry and was scored as 1–2 (negative and weak) or 3–4 (moderate and strong) using a validated scoring schema. Scores 1–2 were considered low EZH2; scores 3–4 were considered high EZH2. In this study, we found that after a median follow up of 9 years (range 0.04–14.5 years) 46 of 480 patients (9.6%) developed distant metastasis. High EZH2 was associated with larger size, high histological grade, negative hormone receptors, and first degree family history of breast and/or ovarian carcinoma. While EZH2 could not predict survival in the entire cohort, high EZH2 was a predictor of disease-specific survival in patients with early-stage disease and first degree family history (log rank P value 0.05). Importantly, in this group of patients, high EZH2 was an independent predictor of distant metastasis up to 15 years after primary carcinoma diagnosis (hazard ratio 6.58, 95% CI: 1.40–30.89, P = 0.016) providing survival information above and beyond currently used prognosticators. In conclusion, EZH2 may be a useful biomarker of long-term metastatic risk in women with familial early-stage breast cancer, and warrant further validation studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of women are diagnosed with node negative invasive breast carcinomas smaller than 2 cm. Even though the majority of patients with small tumors treated with breast conservation have a favorable outcome, the 10-year rate of distant metastasis is up to 20% [1, 2]. As metastatic disease is incurable, accurate prognosticators and more efficacious treatments are needed. Clinical management of patients diagnosed with early-breast cancer is critical but available data on biomarkers of tumor metastasis are limited. This is in part due to the difficulties in developing a cohort of early-stage invasive carcinoma with long-term follow-up and comprehensive pathological, clinical, and treatment information.
Enhancer of Zeste-2 (EZH2) is a Polycomb Group (PcG) protein involved in the control of transcriptional memory and gene silencing which has been shown to exert oncogenic effects in the breast [3–7]. EZH2 methylates histone H3 at lysine 27 (H2K27) thereby recruiting other members of the PcG family to specific genetic target loci [3–7]. It is postulated that EZH2 promotes breast cancer progression by transcriptional repression of tumor suppressors and by maintaining the cells in a stem cell state [8, 9]. We and other investigators have found that EZH2 promotes neoplastic progression in the breast and that EZH2 down-regulation reduces in vivo tumor growth of breast cancer cells [10–12]. EZH2 controls cell proliferation, invasion, and has recently been shown to regulate DNA repair pathways and genomic stability [10, 13–17]. In breast cancer, EZH2 is associated with estrogen (ER) and progesterone (PR) receptor negative status and the available data support the role of EZH2 as a biomarker of breast cancer recurrence [11, 18, 19]. However, whether EZH2 can predict metastasis in the clinically challenging group of early-stage invasive carcinomas is unknown.
In this article, we evaluated the ability of EZH2 expression to predict the risk of metastasis of invasive carcinomas smaller than 2 cm, either node negative or with limited axillary disease (1–3 positive lymph nodes) (Stages I and IIA T1 N1). In this study, we developed a cohort of 480 early-stage invasive breast carcinomas with up to 15 years of follow-up, clinical data, and treatment information.
Methods
Selection of patients and medical record abstraction
Using a managed health system tumor registry, we identified early-stage breast cancer cases diagnosed and treated at Henry Ford Hospital (HFH) between 1996 and 2002 including Stage I and node positive Stage IIA (T1, N1) primary breast carcinomas. The Henry Ford Health System maintains an electronic medical record for each patient. The medical record captures all patient encounters and test results. For each patient identified through the HFH tumor registry, we reviewed the medical record to confirm eligibility. Patients were eligible for the study if they had been diagnosed and treated for a primary, initial invasive breast cancer. Patients were excluded if the primary treatment was not received at HFH, the cancer was bilateral, patient was pregnant at the time of diagnosis, or if there was a prior breast cancer. In this study, we also excluded patients with any other clinically active malignancy. Medical records were reviewed for eligible cases to collect clinical-pathologic and demographic data. Variables abstracted include age at diagnosis, race, family history, parity, age of first live birth, age at menarche, age at menopause, tumor characteristics, treatment received, and tumor recurrence. All aspects of this study were approved by the Institutional Review Boards at Henry Ford Hospital and the University of Michigan.
Microarray construction and immunohistochemistry
A total of 480 primary invasive carcinomas T1 (smaller than 2 cm), node negative, or having 1–3 positive lymph nodes were used for tissue microarray (TMA) construction in triplicate (n = 1,443 tissue microarray samples). Each H&E slide was reviewed by the study pathologist and arrayed in six TMAs as described [11]. Optimally, three 0.4 mm cores were taken from each patient’s sample.
Immunohistochemistry was performed on the TMAs by using a standard biotin–avidin complex technique and by using a standard polyclonal antibody against EZH2 that was previously validated by immunoblot analysis [11, 20]. The TMAs were immunostained for HER-2/neu as performed in clinical practice [21]. At least two authors scored each tumor core blinded to pathological and clinical characteristics. The nuclear EZH2 expression was scored as negative (score = 1, no staining); weak (score 2, <25% of nuclei staining, any intensity); moderate (score = 3, 25–75% of nuclei staining, any intensity); and strong (score = 4, >75% of nuclei staining, any intensity) following previous studies [11, 20]. High EZH2 expression was defined as scores 3 and 4; low EZH2 was defined as scores 1 and 2. Reporting recommendations for tumor marker prognostic studies (REMARK) were followed [22].
Statistical analysis
The a priori planned analysis was to explore the relationship between the EZH2 expression and each clinicopathologic variable available for the cohort. The EZH2 expression was dichotomized into high and low. Since three core samples were obtained for each patient, the highest value of the 3 scores was used for subsequent analysis. For each variable, we assessed the association with EZH2 expression using chi-square and logistic regression. Time to any recurrence and distant metastasis survival curves were constructed by the Kaplan–Meier method. The Cox Proportional Hazards models were utilized to calculated hazard ratios for distant metastasis with 95% confidence intervals. Univariate analyses of time to distant metastasis were performed by using a two-sided log-rank test to evaluate stage, grade, tumor size, nodal status, histology, ER status, PR status, HER-2/neu status, and EZH2. Multivariate associations were modeled using a stepwise modeling approach to identify variables that best fit the model for distant metastasis. Model entrance criteria were P value of 0.25 and model retention was P = 0.15. Hazard ratios and 95% confidence intervals are reported. The analyses were performed for all cases and separately for those with a positive family history of breast and/or ovarian cancer in a first-degree relative.
Results
Patient characteristics
We identified a total of 906 cases through the HFH tumor registry that met the stage and year of diagnosis criteria for inclusion in the study. Of these, 637 were Stage I (<2 cm with negative axillary lymph nodes) and 269 were Stage II (T1 N1) cases (<2 cm with 1–3 positive axillary lymph nodes). After medical record review, 137 (15%) were excluded from the study. Of the 769 eligible cases, tumor specimens were not available for 233 (30%). Of the 536 cases with blocks available, we successfully evaluated the histopathology, immunostained, and scored 480 (90%). Clinical and pathological characteristics as well as the breakdown of treatment modalities are summarized in Table 1.
After a median follow up of 9 years (range 0.04–14.5 years), 80 and 46 of the 480 patients developed any recurrence and distant metastasis (17 and 9.6%, respectively). The 5-, 10- and 15-year recurrence-free survival for the entire cohort of patients were 92, 87, and 85%, respectively. The 5-, 10- and 15-year metastasis-free survival for the entire cohort of patients were 94, 91, and 90%, respectively.
Associations of EZH2 expression and clinical and pathological features of early-stage breast cancer
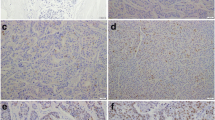
High EZH2 expression was present in 277 of 480 tumors (57.6%). Figure 1a shows representative cases of EZH2 immunohistochemical staining. EZH2 protein was expressed in the nuclei of breast cancer cells as previously reported [11]. The association between EZH2 protein levels with socio-demographic and clinicopathological characteristics is shown in Tables 2 and 3, respectively. EZH2 protein levels were not associated with age at cancer diagnosis, race, age at menarche, or parity. In this study, we found that high EZH2 expression was significantly associated with a family history of breast and/or ovarian cancer in a first-degree relative (odds ratio 1.63, 95% CI: 1.07–2.50, P = 0.02).
a EZH2 expression in early-stage invasive breast carcinomas. Tissue microarray elements containing representative invasive carcinomas with negative (1), weak (2), moderate (3), and strong (4) EZH2 staining intensities. It is noted that EZH2 protein is expressed in the nuclei of cancer cells. Original magnification ×40. b EZH2 protein expression is associated with metastasis-specific survival in patients with early-stage breast cancer and first-degree family history. Kaplan–Meier plot showing that women with tumors expressing high EZH2 have worse metastasis-free survival compared with women with low EZH2 expressing tumors
High EZH2 was associated with tumor size (HR 1.69, CI: 1.13–2.51, P = 0.01), one of the strongest known predictors of survival. High EZH2 expression was also associated with high-histologic grade (HR 4.94, CI: 3.23–7.69, P < 0.0001), a measure of the degree of tumor differentiation and poor prognostic indicator. The high EZH2 expression was associated with ductal tumor histology (HR 2.20, CI: 1.22–4.00, P = 0.008), negative estrogen receptor status (HR 5.96, CI: 3.71–9.59, P < 0.0001), negative progesterone receptor status (HR 3.28, CI: 2.17–4.95, P < 0.0001), and with HER-2/neu overexpression (HR 1.72, CI: 1.11–2.70, P = 0.01).
High EZH2 levels independently predict distant metastasis in early-stage breast cancer patients with first-degree family history
We next tested the hypothesis that EZH2 protein expression may predict the development of distant metastasis in women with early-stage breast cancer and first-degree family history. We focused on this group of women because of recent studies from our laboratory and other investigators showing a mechanistic link between EZH2 and the breast and ovarian cancer tumor suppressor protein BRCA1 [10, 15, 23, 24]. In our cohort of 480 patients, 112 (23%) had a first-degree family history of breast and/or ovarian cancer while 368 (77%) patients had no family history or family history in a non-first degree family member. The characteristics and associations of clinical and pathological features according to the presence or absence of the first-degree family history are shown in Table 4. Patients with first-degree family history were significantly younger, had tumors with higher histological grade, and more frequently estrogen receptor negative than patients with non-first degree family history or no family history. In our cohort, first-degree family history was not associated with tumor size, nodal status, progesterone receptor status, HER-2/neu overexpression, tumor recurrence, or metastasis.
We next sought to determine whether the EZH2 expression could predict metastasis in women with first-degree family history and early-stage breast cancer. Higher tumor stage (I vs. IIA), negative estrogen and negative progesterone receptor status, and high EZH2 expression had significant univariate associations with the development of distant metastasis for patients with first degree family history at 5, 10 and up to 15 years after primary invasive carcinoma diagnosis (Table 5; Fig. 1b). The multivariable model indicates that high EZH2 expression was independently associated with the development of distant metastasis at 5 years (HR 13.04; 95% CI 1.42–119.50, P = 0.023) and up to 15 years following the primary breast carcinoma diagnosis (HR 6.58; 95% CI 1.40–30.9, P = 0.0169) Table 6.
Discussion
In this study, we tested the hypothesis that the expression of EZH2 may be clinically useful in predicting prognosis in a challenging group of patients with invasive carcinomas smaller than 2 cm (T1) with negative lymph nodes (N0) or 1–3 positive nodes (N1) treated by the standard of care between 1996 and 2002. There are few studies in the literature which focus on biomarker development in early-stage breast cancer with limited clinical significance. The identification of patients with early stage biologically aggressive tumors has the potential to impact patient management.
Based on a cohort of 480 early-stage invasive carcinomas of the breast rigorously selected and histopathologically analyzed, we made several novel observations. Consistent with its oncogenic function, the EZH2 overexpression is associated with high-histological grade (Nottingham grade 3) and tumor size, negative estrogen and progesterone receptors, and with HER-2/neu overexpression. In this study, we discovered that the expression of EZH2 is higher in early-stage breast carcinomas from women with first-degree family history of breast and/or ovarian cancer, suggestive of inherited breast cancer susceptibility [25]. Our finding that EZH2 overexpression is associated with familial breast cancers in early-stage disease is especially relevant given previous studies supporting a mechanistic connection between EZH2 and hereditary breast cancer genes BRCA1 and TP53. Our laboratory has shown that EZH2 protein is up-regulated in benign appearing lobules of prophylactic mastectomies from BRCA1 mutation carriers [23, 26]. EZH2 protein is increased in estrogen receptor negative invasive breast carcinomas, where it regulates BRCA1 expression and intracellular localization [10, 15]. The relationship between EZH2 and BRCA1 is likely complex and reciprocal as EZH2 overexpression was detected in breast cancers arising in women with BRCA1 mutations [24]. Pietersen et al. [27] have reported that invasive breast carcinomas with high EZH2 protein harbor TP53 mutations and suggested that during tumorigenesis EZH2 overexpression coincides with TP53 activation. Our results highlight the relevance of these basic studies to human breast cancer and suggest the hypothesis that EZH2 overexpression may be associated with other molecular determinants of hereditary breast cancer in addition to BRCA1 and TP53 gene mutations, which warrant investigation.
Even though most women with early-stage breast cancer in the sporadic or familial settings do not progress, distant metastases may occur long after the first 5 years following primary breast cancer treatment [1]. A major challenge in the management of women with early-stage breast cancer is the identification of those who will develop metastasis from those that will not. In this study, we tested the hypothesis that the EZH2 overexpression in early-stage primary breast carcinoma may predict long-term metastasis up to 15 years following diagnosis of the primary tumor. Indeed, EZH2 expression levels in primary breast carcinoma were associated with metastasis-specific survival in women with first-degree family history at 5, 10 and up to 15 years after primary breast carcinoma diagnosis. The 15-year-metastasis-free survival was 83 and 94% for women with tumors exhibiting high and low EZH2 expression, respectively (log rank test P = 0.05). The best multivariable model predictive of metastasis-specific survival included high EZH2 expression, high histological grade, higher stage, and absence of anti-hormonal treatment, reflecting negative estrogen and progesterone receptor status. High EZH2 expression was a strong independent predictor of metastasis providing 15-year survival information above other independent prognostic features. These data are clinically relevant because EZH2 may identify women with early-stage breast cancer and first-degree family history at higher risk to develop distant metastasis. Furthermore, our data support the potential clinical utility of incorporating EZH2 into clinical nomograms to help determine the risk of cancer progression in this specific group of patients.
A limitation of our analysis is that tumor tissue samples were not available for 30% of patients identified. However, given that these samples would have not been selected on EZH2 expression, it is unlikely to have biased our results. Our study focused on small tumors so the material resource was very limited. Despite this, we successfully utilized 90% of the available tissues in our analysis. In this study, our findings were in part based on a small group of women in our cohort (n = 112 out of 480) who had first-degree family history of breast and/or ovarian cancer. Therefore, some of the results included large confidence intervals. Nonetheless, we were able to demonstrate a significant association of EZH2 with distant metastasis even after controlling for important covariates, which strengthens our results.
Since our initial reports of EZH2 overexpression in breast cancer, our findings have been supported by other investigations. EZH2 overexpression has been found in breast and other solid tumors including bladder [28–30], gastric [31], lung [32], and hepatocellular carcinoma [33], as well as in melanoma [17]. In these malignancies, EZH2 has been implicated in neoplastic transformation, progression, invasion, and metastasis. Taken together, these data suggest that EZH2 may be involved in a global, rather than a tissue type specific mechanism of tumor progression.
In conclusion, our results indicate that in early-stage breast cancer, EZH2 overexpression is associated with features of aggressive disease. In this study, we discovered a novel association between the overexpression of EZH2 and a first-degree family history of breast and/or ovarian cancer, which paves the way to mechanistic and functional studies. Our finding that EZH2 overexpression is an indicator of metastasis and metastasis-related deaths in women with early-stage breast cancer and first-degree family history may have important clinical implications. Specifically, EZH2 detection at the time of primary tumor diagnosis may aid clinicians in guiding management decisions and lays the foundation for the development of targeted treatments.
References
Sarrazin D, Dewar JA, Arriagada R et al (1986) Conservative management of breast cancer. Br J Surg 73:604–606
Llombart-Cussac A (2008) Improving decision-making in early breast cancer: who to treat and how? Breast Cancer Res Treat 112(Suppl 1):15–24
Laible G, Wolf A, Dorn R et al (1997) Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J 16:3219–3232
Satijn DP, Otte AP (1999) Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta 1447:1–16
Ringrose L, Paro R (2004) Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet 38:413–443
Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM (1991) Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65:753–763
Hess JL (2004) MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med 10:500–507
Boyer LA, Plath K, Zeitlinger J et al (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441:349–353
Levine SS, Weiss A, Erdjument-Bromage H, Shao Z, Tempst P, Kingston RE (2002) The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol Cell Biol 22:6070–6078
Gonzalez ME, Li X, Toy K et al (2009) Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 28:843–853
Kleer CG, Cao Q, Varambally S et al (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 100:11606–11611
Cao Q, Yu J, Dhanasekaran SM et al (2008) Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27:7274–7284
Zeidler M, Varambally S, Cao Q et al (2005) The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia 7:1011–1019
Chang CJ, Yang JY, Xia W et al (2011) EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell 19:86–100
Gonzalez ME, DuPrie ML, Krueger H, Merajver SD, Ventura AC, Toy KA, Kleer CG (2011) Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res 71:2360–2370
Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K (2003) EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 22:5323–5335
Bachmann IM, Halvorsen OJ, Collett K et al (2006) EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 24:268–273
Collett K, Eide GE, Arnes J et al (2006) Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 12:1168–1174
Raaphorst FM, Meijer CJ, Fieret E et al (2003) Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 5:481–488
Varambally S, Dhanasekaran SM, Zhou M et al (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629
Gown AM (2008) Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol 21(Suppl 2):S8–S15
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG (2006) Identification of EZH2 as a molecular marker for a precancerous state in morphologically normal breast tissues. Cancer Res 66:4095–4099
Puppe J, Drost R, Liu X et al (2009) BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer 11:R63
Chabner E, Nixon A, Gelman R et al (1998) Family history and treatment outcome in young women after breast-conserving surgery and radiation therapy for early-stage breast cancer. J Clin Oncol 16:2045–2051
Ding L, Kleer CG (2006) Enhancer of Zeste 2 as a marker of preneoplastic progression in the breast. Cancer Res 66:9352–9355
Pietersen AM, Horlings HM, Hauptmann M et al (2008) EZH2 and BMI1 inversely correlate with prognosis and TP53 mutation in breast cancer. Breast Cancer 10:R109
Arisan S, Buyuktuncer ED, Palavan-Unsal N, Caskurlu T, Cakir OO, Ergenekon E (2005) Increased expression of EZH2, a polycomb group protein, in bladder carcinoma. Urol Int 75:252–257
Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ (2005) Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 11:8570–8576
Weikert S, Christoph F, Kollermann J et al (2005) Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med 16:349–353
Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H (2006) Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci 97:484–491
Breuer RH, Snijders PJ, Smit EF et al (2004) Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia 6:736–743
Sudo T, Utsunomiya T, Mimori K et al (2005) Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 92:1754–1758
Acknowledgments
The authors thank Mrs. Robin Kunkel for artwork and Javed Siddiqui for his assistance with tissue microarray construction. This study was financially supported by the National Cancer Institute at the National Institutes of Health (R01 CA125577, UO1 CA154224, and 2R01 CA107469) to CGK; the Department of Defense Breast Cancer Research Program to SHA; the University of Michigan Comprehensive Cancer Center Core Grant (NIH/NCI 5 P30 CA46592); and the Josephine Ford Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alford, S.H., Toy, K., Merajver, S.D. et al. Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat 132, 429–437 (2012). https://doi.org/10.1007/s10549-011-1591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1591-2